State Pharmacopoeia of the Russian Federation xiii edition. Determination of the main groups of biologically active substances
Medicines are a specific production product, the quality of which the consumer cannot evaluate independently. Guaranteeing the quality of both medicines produced in Russia and imported from abroad is one of the main tasks of the state in the field of protecting public health. The most important tasks include not only saturating the domestic pharmaceutical market with these medicines, but also entering the international pharmaceutical market, which can be achieved by ensuring that domestic medicines comply with the requirements of world standards, noted Minister Veronika Skvortsova in her introductory remarks to the publication.
The new edition of the State Pharmacopoeia, XIII edition, solves these strategic problems.
The first pharmacopoeia, published in 1765, was in Latin and was intended to ensure the quality of medicines used by surgeons in military hospitals. In subsequent years, decades and centuries, the domestic pharmacopoeia was repeatedly republished, updating its content in accordance with the state and level of development of the pharmaceutical industry and the control and licensing system both in our country and abroad, said the director of the Department of State Regulation of Medicines Circulation in his introduction. funds Arsalan Tsyndymeev.
The State Pharmacopoeia of the XIII edition included 229 general pharmacopoeial articles and 179 pharmacopoeial articles.
For the first time, 99 general pharmacopoeial articles are introduced into the State Pharmacopoeia of the XIII edition, including 30 on methods of analysis, 5 on dosage forms and 12 on methods for determining pharmaceutical and technological parameters of dosage forms. In addition, 2 general pharmacopoeial articles - on medicinal plant raw materials and 3 - on methods of its analysis, 7 - on groups of immunobiological drugs and 31 - on methods of their testing, 3 - on groups of drugs from blood and blood plasma of humans and animals, 9 - on methods of analysis of medicinal products obtained from blood and blood plasma of humans and animals.
The main goal pursued by the State Pharmacopoeia of the Russian Federation is to standardize the quality of medicines in circulation on the domestic pharmaceutical market, notes Elena Sakanyan, director of the Center for Pharmacopoeia and International Cooperation of the Federal State Budgetary Institution “NTsESMP”.
The timely release of the State Pharmacopoeia of the Russian Federation, which, in accordance with the Federal Law “On the Circulation of Medicines” should be carried out with a frequency not exceeding 1 time in 5 years, will be the key to solving the problem of ensuring high-quality medical care provided to the population of our country.
Order of the Ministry of Health of the Russian Federation dated October 29, 2015 No. 771 provides for the introduction into force of general pharmacopoeial monographs and pharmacopoeial monographs included in the State Pharmacopoeia of the XIII edition from January 1, 2016.
It has been established that general pharmacopoeial monographs and pharmacopoeial monographs approved by this order, general pharmacopoeial monographs and pharmacopoeial monographs approved by order of the Ministry of Health of the Russian Federation dated November 21, 2014 No. 768 “On approval of general pharmacopoeial monographs and pharmacopoeial monographs” constitute the State Pharmacopoeia XIII publications
Determined that:
regulatory documentation for registered medicinal products for medical use, as well as for medicinal products for medical use, applications for state registration of which are submitted to the Ministry of Health of the Russian Federation before the entry into force of the pharmacopoeial monographs approved by this order, shall be brought into conformity with these pharmacopoeial monographs before January 1, 2018;
regulatory documentation for registered medicinal products for medical use, as well as for medicinal products for medical use, applications for state registration of which are submitted to the Ministry of Health of the Russian Federation before the entry into force of the general pharmacopoeial monographs approved by this order, shall be brought into conformity with these general pharmacopoeial monographs articles until January 1, 2019.
The new State Pharmacopoeia can be found at the following email address:
A feature of the current stage of standardization of medicines is the need to harmonize the requirements for the quality of medicines and their testing methods imposed by the Russian Pharmacopoeia and leading foreign pharmacopoeias.
The XII edition of the State Pharmacopoeia of the Russian Federation will include five parts.
The first part describes general provisions, methods of analysis, requirements for pharmaceutical substances, and pharmacopoeial monographs for the substance.
The State Pharmacopoeia (SP) is a collection of basic standards used in pharmacopoeial analysis and production of medicines. The state pharmacopoeia has a legislative nature. The basis of the State Pharmacopoeia is made up of general pharmacopoeial monographs (GPM) and pharmacopoeial monographs (FS). The General Pharmacopoeial Standard describes the general provisions and methods of analysis adopted in pharmacopoeial analysis, or includes a list of standardized indicators and test methods for a particular dosage form. The FS determines the level of requirements for specific medicines.
CONTENT
I. EDITORIAL BOARD OF ROSZDRAVNADZOR ON THE ORGANIZATION OF WORK ON THE STATE PHARMACOPOEIA 7
II. PREFACE 9
III. ORGANIZATIONS, INSTITUTIONS OF RUSSIA AND SPECIALISTS WHO TOOK PART IN THE PREPARATION OF PART 1 OF THE STATE PHARMACOPOEIA OF THE RUSSIAN FEDERATION XII EDITION 10
IV. INTRODUCTION 13
GENERAL PHARMACOPOEIAL ARTICLES
1. Rules for the use of pharmacopoeial monographs (OFS 42-0031-07) 17
2. International system (SI) units used in the pharmacopoeia and their correspondence to other units (OFS 42-0032-07) 22
ANALYSIS METHODS 26
3. Equipment (OFS42-0033-07) 26
PHYSICAL AND PHYSICAL-CHEMICAL METHODS OF ANALYSIS 29
4. Melting point (OFS 42-0034-07) 29
5. Solidification temperature (OFS 42-0035-07) 34
6. Temperature limits of distillation and boiling point (OFS 42-0036-07) 36
7. Density (OFS 42-0037-07) 38
8. Viscosity (OFS 42-0038-07) 41
9. Determination of ethyl alcohol in liquid pharmaceutical preparations (OFS 42-0039-07) 49
10. Refractometry (OFS 42-0040-07) 52
11. Polarimetry (OFS 42-0041 -07) 54
12. Spectroscopic methods 56
12.1. Spectrophotometry in the ultraviolet and visible regions (OFS 42-0042-07) 56
12.2. Spectrometry in the infrared region (OFS 42-0043-07) 62
12.3. Atomic emission and atomic absorption spectrometry (OFS 42-0044-07) 66
12.4. Fluorimetry (OFS 42-0045-07) 70
12.5. Nuclear magnetic resonance spectroscopy (OFS42-0046-07) 73
13. Osmolarity (OFS 42-0047-07) 78
14. Ioiom&trt (OFS 42-0048-07) 85
15. Solubility (OFS 42-0049-07) 92
16. Color degree of liquids (OFS 42-0050-07) 93
17. Transparency and degree of turbidity of liquids (OFS 42-0051-07) 98
CHEMICAL METHODS OF ANALYSIS 101
18. Determination of nitrogen in organic compounds by the Kjeldahl method (OFS 42-0052-0 7) 101
19. Determination of protein (OFS 42-0053-07) 105
20. Nitritometry SOFS 42-0054-0 7) 114
IMPURITY LIMIT TEST 115
21. Total ash (OFS 42-0055-07) 115
22. Sulfated ash (OFS 42-0056-07) 115
23. Residual organic solvents (OFS 42-0057-07) 115
24. Test for purity and permissible limits of impurities 118
24.1. Iron (OFS 42-0058-07) 119
24.2. Heavy metals (OFS 42-0059-07) 121
BIOLOGICAL CONTROL METHODS 124
25. Abnormal toxicity (OFS 42-0060-07) 124
26. Pyrogenicity (OFS 42-0061-07) 125
27. Bacterial endotoxins (OFS 42-0062-07) 128
28. Histamine test (OFS 42-0063-07) 136
29. Test for depressant substances (OFS 42-0064-07) 140
30. Biological methods for assessing the activity of medicinal plant materials and drugs containing cardiac glycosides (OFS 42-0065-07) 141
31. Sterility (OFS 42-0066-0 7) 150
32. Microbiological purity (OFS 42-0067-07) 160
33. Determination of the antimicrobial activity of antibiotics by diffusion into agar (OPS 42-0068-0 7) 194
34. Determination of the effectiveness of antimicrobial preservatives for drugs (OFS 42-0069-07) 216
REAGENTS 220
35. Reagents. Indicators (OFS 42-0070-07) 220
36. Titrated solutions (OFS 42-0071-07) 425
37. Buffer solutions (OFS 42-0072-07) 443
38. Radiopharmaceuticals (OFS 42-0073-07) 456
39. Pharmaceutical substances (OFS 42-0074-07) 484
40. Shelf life of medicines (OFS 42-0075-07) 488
PHARMACOPOEIAL ARTICLES 493
Download the e-book for free in a convenient format, watch and read:
Download the book State Pharmacopoeia of the Russian Federation, XII edition, Part 1, 2007 - fileskachat.com, fast and free download.
Download file No. 1 - doc
Download file No. 2 - djvu
Below you can buy this book at the best price with a discount with delivery throughout Russia.
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
PHARMACOPOEIAL ARTICLE
GinsengpresentrootsFS.2.5.0013.15
Panacis ginseng radices In return for the Global FundXI, vol. 2, art. 66
Collected in late August - early September and dried roots of the wild and cultivated perennial herbaceous plant true ginseng - Panax ginseng C. A. Mey, sem. Araliaceae – Araliaceae.
AUTHENTICITY
External signs. Whole raw materials. Roots up to 25 cm long, 0.7–2.5 cm thick, with 2–5 large branches, less often without them. The roots are taprooted, longitudinally, less often spirally wrinkled, fragile, with an even fracture. The “body” of the root is thickened, almost cylindrical, with clearly defined annular thickenings on top. In the upper part of the root there is a narrowed transversely wrinkled rhizome - a “neck”. The rhizome is short with several scars from fallen stems; at the top it forms a “head”, which is an expanded remainder of the stem and an apical bud (sometimes 2–3). One or more adventitious roots sometimes extend from the “neck”. The “neck” and “head” may be missing. The color of the roots on the surface and on the cut is yellowish-white, on a fresh fracture it is white. The smell is specific. The taste of the water extract is sweet, pungent, then spicy-bitter.
Crushed raw materials. When examining crushed raw materials under a magnifying glass (10×) or a stereo microscope (16×), pieces of roots of various shapes are visible passing through a sieve with 7 mm holes. The color on the surface and on the fracture is yellowish-white. The smell is specific. The taste of the water extract is sweet, pungent, then spicy-bitter.
Powder. When examining the powder under a magnifying glass (10×) or a stereo microscope (16×), a mixture of crushed particles of roots of various shapes of a yellowish-white color is visible, passing through a sieve with 2 mm holes. The smell is specific. The taste of the water extract is sweet, pungent, then spicy-bitter.
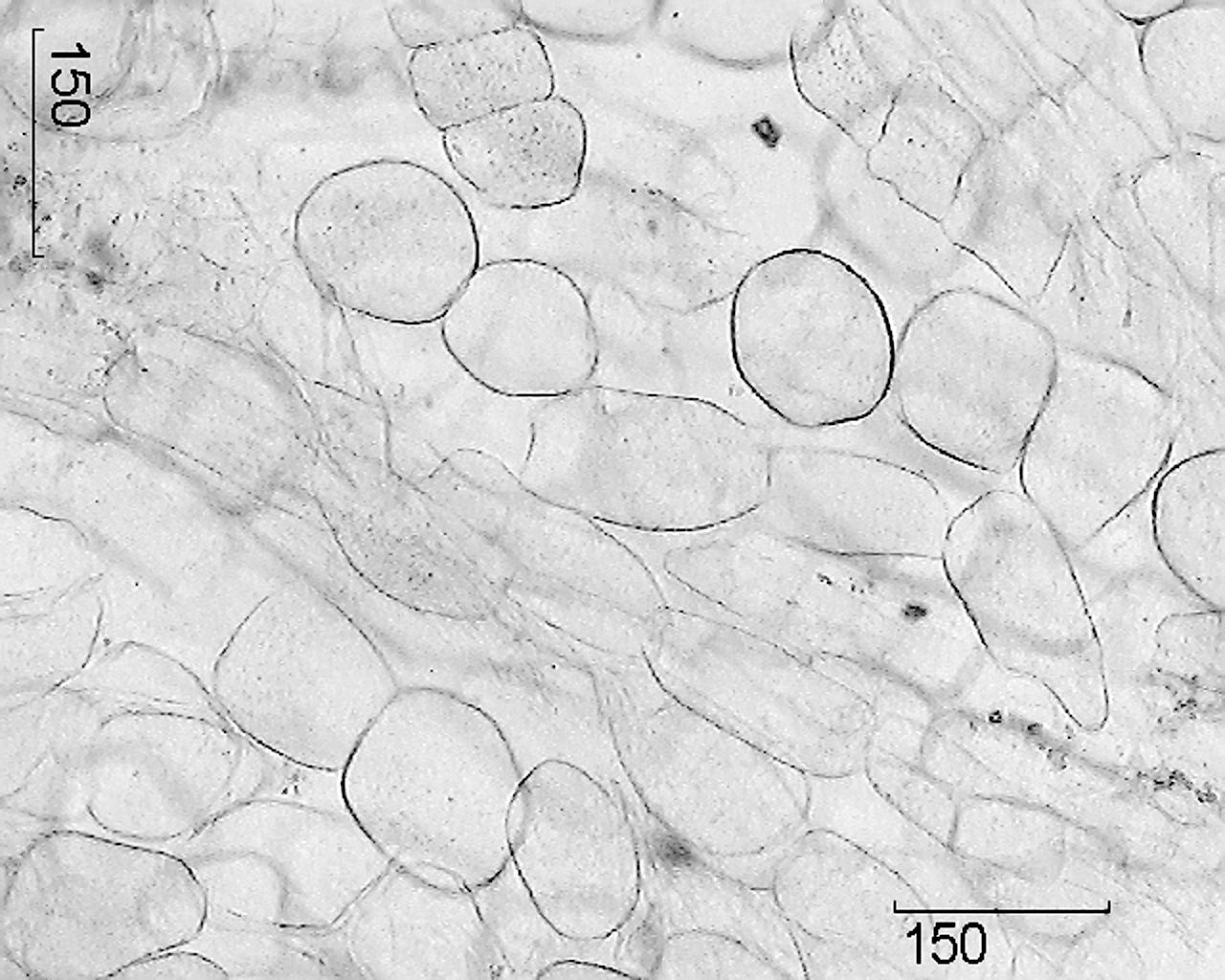
Microscopic signs. Whole raw materials. A cross section of the main root reveals a narrow layer of light brown plug, wide bark, a clear cambium line and wood.
The main root is covered with periderm, the cells of which are thin-walled and lignified, non-suberized. Phloem and xylem are separated by the cambial zone, which runs approximately through the middle of the root radius and
sometimes it is not visible. To the periphery, large-celled primary radial rays of parenchyma tissue extend from the primary xylem, between which there is secondary xylem, intersected by numerous secondary radial rays of the main parenchyma. Xylem consists of thin-walled parenchyma cells containing starch grains. The vessels of the medullary rays have thickened, lignified walls and are located singly or collected in groups of 3–6. Cells containing yellow pigments are occasionally found in the wood parenchyma. In the center of the root there are vaguely recognizable remains of primary xylem in the form of 2 rays. Phloem consists mainly of small-celled elements; it contains clearly visible schizogenic containers containing droplets of secretion from light yellow to red-brown. Starch grains are small, round, simple. Individual parenchyma cells contain drusen of calcium oxalate. The outer part of the secondary cortex is bordered by a zone of several (4–6) rows of large tangentially elongated parenchyma cells of the phelloderm, round or oval, with a slightly thickened shell.
|
|
||
|
|
|
|
|
|
|
|
Picture – Real ginseng roots.
1 – fragment of a cross section of the main root (100×); 2 – cork fragment (400×); 3 – fragment of a cross section of an adventitious root: a – xylem vessels, b – starch grains (400×); 4 – fragment of a cross section of the main root with a secretory canal: a – lining cells of the canal, b – canal cavity (400×); 5 – fragment of the parenchyma of the medullary rays: a – calcium oxalate drusen, b – starch grains (400×); 6 – parenchyma cells of the medullary ray (100×).
On a cross section of an adventitious root, in the center, a ray of vessels of the primary xylem is the remnant of the diarchic vascular bundle in the primary structure. Two sectors of secondary xylem are separated by radial rays of the main parenchyma. Parenchyma cells are round or oval, partially or completely filled with starch grains. The cork consists of 5–7 layers of rectangular, thin-walled cells, weakly lignified.
Crushed raw materials. When examining a pressed specimen, fragments of transverse and longitudinal sections of the main and adventitious roots should be visible.
Fragments of the main root are represented by xylem rays and vessels, filling parenchyma cells of the medullary rays with starch grains, canal cavities and lining cells, parenchyma cells with pigments, and cambium cells.
Fragments of the adventitious root are represented by plug cells, parenchyma with starch grains, receptacles, primary and secondary cortex, vessels, medullary rays.
Powder. When examining a microslide, fragments of the epidermis, cork, wood, parenchyma, as well as drusen of calcium oxalate are visible.
Determination of the main groups of biologically active substances
Thin layer chromatography
On the starting line of an analytical chromatographic plate with a layer of silica gel with a fluorescent indicator measuring 10 × 15 cm on an aluminum substrate, apply 20 μl of the test solution (see section “Quantitative determination”, preparation of solution A of the test solution) and 50 μl of a standard sample solution (SS) of panaxoside Rg 1 (see section “Quantitative determination” preparation of solution A CO panaxoside Rg 1). The plate with the applied samples is dried in air, placed in a chamber, pre-saturated for at least 2 hours with a solvent mixture of chloroform - methanol - water (26:14:3), and chromatographed using an ascending method. When the solvent front passes about 80–90% of the length of the plate from the starting line, it is removed from the chamber, dried until traces of solvents are removed, treated with phosphotungstic acid with a 20% alcohol solution and heated in an oven at 100–105 °C for 3 minutes, after which is viewed in daylight.
The chromatogram of the test solution should show at least 6 adsorption zones from light pink to dark pink; the dominant zone is at the zone level in the chromatogram of the CO solution of panaxoside Rg 1 ; detection of other adsorption zones is allowed.
When a drop of concentrated sulfuric acid is applied to the ginseng root powder after 1–2 minutes, a brick-red color appears, turning into red-violet, and then violet (panaxosides).
Tests
Humidity. Whole raw materials crushed raw materials, powder – no more than 13%.
Common ash. Whole raw materials crushed raw materials, powder – no more than 5%.
Ash, insoluble in hydrochloric acid. Whole raw materials crushed raw materials, powder – no more than 2%.
Grinding of raw materials.Whole raw materials: particles passing through a sieve with holes measuring 3 mm - no more than 5%. Crushed raw materials: particles that do not pass through a sieve with holes measuring 7 mm - no more than 5%; particles passing through a sieve with holes measuring 0.5 mm - no more than 5%. Powder: particles that do not pass through a sieve with holes measuring 2 mm - no more than 5%; particles passing through a sieve with holes measuring 0.18 mm - no more than 5%.
Foreign matter
Roots darkened from the surface . Whole raw materials crushed raw materials – no more than 3%.
Organic impurity. Whole raw materials crushed raw materials – no more than 0.5%.
Mineral impurity . Whole raw materials, crushed raw materials, powder – no more than 1%.
Heavy metals. In accordance with the requirements of the General Pharmacopoeia Monograph “Determination of the content of heavy metals and arsenic in medicinal plant materials and medicinal herbal preparations.”
Radionuclides. In accordance with the requirements of the General Pharmacopoeia Monograph “Determination of radionuclide content in medicinal plant materials and medicinal herbal preparations.”
Pesticide residues. In accordance with the requirements of the General Pharmacopoeia Monograph “Determination of the content of residual pesticides in medicinal plant materials and medicinal herbal preparations.”
Microbiological purity. In accordance with the requirements of the General Pharmacopoeia Monograph “Microbiological purity”.
quantitation. Whole raw materials crushed raw materials, powder: the amount of panaxosides in terms of panaxoside Rg 1 - not less than 2%; extractive substances extracted with 70% alcohol - at least 20%.
(“State Pharmacopoeia of the Russian Federation. XIII edition. Volume I”)
This general pharmacopoeial article establishes general requirements for the storage of pharmaceutical substances, excipients and medicinal products and applies to all organizations in which the storage of medicinal products takes place, taking into account the type of activity of the organization.
Storage of medicinal plant raw materials and medicinal herbal preparations is carried out in accordance with the General Pharmacopoeia Monograph “Storage of medicinal plant raw materials and medicinal herbal preparations”.
Storage- the process of storing medicines until they are used within the established expiration date, which is an integral part of the circulation of medicines.
General requirements for storage facilities
medicines and organization of their storage
Storage of medicines must be carried out in premises intended for these purposes. The design, composition, dimensions of storage areas, their operation and equipment must ensure proper storage conditions for various groups of medicines.
The complex of storage facilities should include:
- a receiving room (area) intended for unpacking and receiving packages of medicines and their preliminary inspection;
- room (zone) for sampling medicinal products in accordance with the requirements of the General Pharmacopoeia Monograph “Sampling”;
- room (zone) for quarantine storage of medicines;
- premises for medicines requiring special storage conditions;
- room (area) for storing rejected, returned, recalled and/or expired medications. The specified medications and their storage locations must be clearly marked.
The storage area is allocated in a common storage room in the absence of a separate isolated room.
The finishing of premises for storing medicines must meet current sanitary and hygienic requirements; the internal surfaces of walls and ceilings must be smooth, allowing for wet cleaning.
In each storage room it is necessary to maintain the climatic conditions, observing the temperature and humidity established by the pharmacopoeial monograph or regulatory documentation for medicines.
The necessary air exchange in storage rooms is created using air conditioners, supply and exhaust ventilation or other equipment. Natural and artificial lighting in storage rooms must ensure the accurate and safe implementation of all operations performed in the room. If necessary, protection of medicinal products from solar radiation should be provided.
Premises for storing medicines must be equipped with the required number of duly verified measuring instruments (thermometers, hygrometers, psychrometers, etc.) for monitoring and recording temperature and humidity, carried out at least once a day.
Measuring instruments are placed at a distance of at least 3 meters from doors, windows and heating devices in a place accessible for reading readings, at a height of 1.5-1.7 meters from the floor. At the same time, they are recommended to be placed in places where there is the greatest likelihood of temperature and humidity fluctuations or deviations from the required parameters are most often observed.
Registration records must demonstrate the temperature and humidity conditions established for the premises, and, if they do not comply, corrective actions.
Storage premises must be equipped with a sufficient number of cabinets, safes, racks, storage units, and pallets. Equipment must be in good condition and clean.
Shelving, cabinets and other equipment must be installed in such a way as to ensure access to medicines, free passage of personnel and, if necessary, accessibility of loading and unloading operations, as well as accessibility of equipment, walls, and floors of the room for cleaning.
Proper sanitary conditions must be maintained in the premises for storing medicines. The frequency and methods of cleaning premises must comply with the requirements of regulatory documents. The sanitary disinfectants used must be safe; the risk of contamination of stored medicines with these products must be eliminated.
Specific instructions for cleaning up spilled or scattered medicinal products should be developed to ensure complete elimination and prevent contamination of other medicinal products.
When performing work in premises for storing medicines, employees must wear special clothing and shoes and observe personal hygiene rules.
In storage rooms, medicinal products are placed in accordance with the storage conditions specified in the pharmacopoeial monograph or regulatory documentation for medicinal products, taking into account their physicochemical and hazardous properties, pharmacological and toxicological effects, the type of dosage form of the medicinal product and the method of its use, aggregate state of the drug. When using computer technology, it is allowed to place medicines alphabetically, by code.
Racks, cabinets, and shelves intended for storing medicines must be identified. It is also necessary to identify stored medicines using a rack card, or, when using computer technology, using codes and electronic devices.
When unloading and loading operations are carried out manually, the stacking height of medicines should not exceed 1.5 meters. When using mechanized devices during unloading and loading operations, medicines should be stored in several tiers. At the same time, the total height of placement of medicines on the racks should not exceed the capabilities of loading and unloading mechanisms.
Medicines in storage rooms must be placed in cabinets, racks, shelves, pallets, etc. It is not allowed to place medicines on the floor without a pallet.
Pallets can be placed on the floor in one row or on racks in several tiers, depending on the height of the rack. It is not allowed to place pallets with medicines in several rows in height without the use of racks.
When creating storage conditions for a particular medicinal product, it is necessary to be guided by the requirements specified in the pharmacopoeial monograph or regulatory documentation for this medicinal product, established by the manufacturer (developer) of the medicinal product based on the results of a stability study in accordance with the General Pharmacopeia Monograph “Shelf Life of Medicinal Products”.
Medicines are stored in packaging (consumer, group) that meets the requirements of regulatory documentation for this medicine.
Medicines are stored at a relative humidity of no more than 60%+/-5% depending on the relevant climatic zone (I, II, III, IVA, IVB), unless special storage conditions are specified in the regulatory documentation.
Medicines should be stored to prevent contamination, mixing and cross-contamination. It is necessary to avoid foreign odors in storage areas.
A system for recording medicines with a limited expiration date must be implemented in the organization. If several batches of the same drug name are in storage, then the drug whose expiration date expires earlier than the others should be taken first for use.
Rejected medicinal products must be identified and stored in an appropriate room (area) under conditions that do not allow their unauthorized use.
Features of storage of certain groups of medicines
Medicines with dangerous properties (flammable, explosive, radiopharmaceutical, caustic, corrosive, compressed and liquefied gases, etc.) should be stored in specially designed rooms equipped with additional safety and security equipment.
During storage, it is necessary to ensure the safety and declared quality of medicines, to prevent the possibility of medicines displaying their dangerous properties and to create safe working conditions for employees working with such medicines.
When arranging premises and organizing the storage of dangerous medicines, it is necessary to be guided by the requirements of federal laws and regulatory legal acts of the Russian Federation.
Storage of narcotic and psychotropic medicines must be carried out in accordance with federal laws and regulations of the Russian Federation.
When storing medicines that require protection from the influence of environmental factors (light, temperature, atmospheric composition of air, etc.), it is necessary to ensure the storage regime specified in the pharmacopoeial monograph or regulatory documentation. Deviations from the regulated conditions are allowed once only for a short period (no more than 24 hours), unless special conditions, for example, permanent storage in a cold place, are specified separately.
Medicines that can change their properties under the influence of light energy (oxidize, reduce, decompose, change color, etc.) are photo- or light-sensitive; drugs that are resistant to light are photostable. The influence of light energy can manifest itself in exposure to direct sunlight, scattered light in the visible region of the light spectrum and radiation in the ultraviolet region.
Labeling of photosensitive drugs usually contains the instruction: “Store in a place protected from light.” Medicines that require protection from light should be stored in rooms or specially equipped areas that provide protection from natural and artificial light.
Pharmaceutical substances that require protection from light should be stored either in packaging made from light-protective materials or in a dark room or cabinet. If glass containers for medicines are used to package pharmaceutical substances that are particularly sensitive to light, the container must be covered with black light-proof paper.
Photosensitive medicinal products should be packaged in light-protective secondary (consumer) packaging and/or should be stored in a place protected from light.
Medicines that, when in contact with water, moisture, can release gases, etc., are moisture-sensitive. Labeling of moisture-sensitive medicines usually contains the instruction: “Store in a dry place.”
When storing such medicinal products, it is necessary to ensure that the relative humidity does not exceed 50% at room temperature (under normal storage conditions) or equivalent vapor pressure at another temperature. Fulfillment of the requirement also provides for the storage of a moisture-sensitive medicinal product in airtight (moisture-proof) consumer packaging that provides the specified protection and compliance with storage conditions during circulation of the medicinal product.
To maintain a low moisture content during storage of medicinal products, drying agents are used in prescribed cases, provided that their direct contact with the medicinal product is avoided.
Medicines with hygroscopic properties must be stored at a relative humidity of no more than 50% in packaging, which is a glass container for medicines, hermetically sealed, or in packaging with additional protection, for example, in a plastic film bag, in accordance with the requirements of the pharmacopoeial monograph or regulatory documentation.
Some groups of drugs change their properties under the influence of atmospheric gases, such as oxygen or carbon dioxide. To ensure the protection of medicines from the effects of gases, it is recommended that medicines be stored in sealed packaging made of materials impermeable to gases. If possible, the packaging should be filled to the top and sealed tightly.
Medicines that are actually volatile medicines or medicines containing a volatile solvent; solutions and mixtures of volatile substances; Medicines that decompose with the formation of volatile products require storage conditions that protect them from volatilization and drying out. It is recommended to store medicinal products in a cool place, in hermetically sealed packaging made of materials impermeable to volatile substances, or in primary and secondary (consumer) packaging in accordance with the requirements specified in the pharmacopoeial monograph or regulatory documentation.
Medicines, which are pharmaceutical substances containing water of crystallization (crystal hydrates), exhibit the properties of hygroscopic substances. It is recommended to store crystalline hydrates in hermetically sealed packaging in accordance with the requirements specified in the pharmacopoeial monograph or regulatory documentation. As a rule, crystalline hydrates are stored at temperatures from 8 to 15 ° C and relative air humidity of no more than 60%.
Medicines that change their properties under the influence of ambient temperature are thermosensitive. Medicines can change their properties when exposed to room temperature or higher (heat-labile medicines) or when exposed to low temperatures, including freezing.
When storing heat-sensitive drugs, it is necessary to ensure the temperature regime regulated by the requirements of the pharmacopoeial monograph or regulatory documentation indicated on the primary and/or secondary (consumer) packaging of the drug.
Heat-labile medicines should be stored in specially equipped rooms (refrigerators) or in storage rooms equipped with a sufficient number of refrigerated cabinets and refrigerators. Pharmaceutical refrigerators or refrigerators for blood and blood products should be used to store thermolabile drugs.
The proper quality of immunobiological drugs, the safety and effectiveness of their use is ensured by the “cold chain” system, which must be implemented at all four levels.
Resolution of the Chief State Sanitary Doctor of the Russian Federation No. 15 of April 10, 2002 “On the implementation of sanitary and epidemiological rules SP 3.3.2.1120-02″
Refrigerators (chambers, cabinets) must be set at a temperature that corresponds to the temperature conditions for storing the medicines contained in them. Immunobiological medicinal products should be stored at a temperature not exceeding 8 °C. Each package of immunobiological medicinal product in the refrigerator must be provided with access to cooled air. Immunobiological medicinal products should not be stored together in the refrigerator with other medicinal products.
To monitor the temperature conditions of storage of thermolabile medicines, all refrigerators (chambers, cabinets) must be equipped with thermometers. Continuous monitoring of the temperature regime is carried out using thermographs and temperature recorders, the readings of which are recorded at least twice a day.
The temperature regime on the refrigerator shelves is different: the temperature is lower near the freezer compartment, higher near the opening door panel.
Providing a cold place means storing medicines in the refrigerator at a temperature of 2 to 8 ° C, avoiding freezing. Cool storage means storing medications at a temperature of 8 to 15 °C. In this case, it is allowed to store medicines in the refrigerator, with the exception of medicines that, when stored at a refrigerator temperature below 8 ° C, can change their physical and chemical characteristics, for example, tinctures, liquid extracts, etc.
Storage at room temperature implies a temperature range of 15 to 25 °C or, depending on climatic conditions, up to 30 °C. Storage in the freezer ensures the temperature of medicines from -5 to -18 °C. Deep freezing storage requires temperatures below -18 °C.
It is advisable to place medicines in areas and on refrigerator shelves that correspond to their temperature storage conditions. It is not allowed to store immunobiological drugs on the door panel of the refrigerator.
In storage rooms, it is necessary to provide storage conditions for medicinal products that require protection from exposure to low temperatures, for which the pharmacopoeial monograph or regulatory documentation sets a lower temperature limit for storage.
It is not allowed to freeze medicinal products that have the appropriate requirements in the pharmacopoeial monograph or regulatory documentation and are indicated on the primary or secondary packaging, including insulin preparations, adsorbed immunobiological preparations, etc.
It is not allowed to freeze medicines placed in packaging that can be destroyed by freezing, for example, medicines in ampoules, glass bottles, etc.
The definitions used in the pharmacopoeia that characterize the temperature conditions for storing medicines are given in the table.
It is necessary to ensure compliance with the storage conditions of medicines and maintaining their integrity during transportation.
For medicinal products that are particularly sensitive to changes in temperature (vaccines, serums and other immunobiological medicinal products, insulin medicinal products, etc.), the temperature regime regulated by the pharmacopoeial monograph or regulatory documentation must be observed during transportation.
Definitions characterizing drug storage modes
General pharmacopoeial monographs and pharmacopoeial monographs of the State Pharmacopoeia of the Russian Federation
XIII edition
V.A. Merkulov, E.I. Sakanyan, T.B. Shemeryankina, O.A. Mochikina, N.D. Bunyatyan
Federal State Budgetary Institution "Scientific Center for Expertise of Medical Products" of the Ministry of Health of the Russian Federation, 127051, Moscow, Russia
Summary: The State Pharmacopoeia of the Russian Federation is a set of general pharmacopoeial articles and pharmacopoeial articles and is subject to reissue at least once every 5 years. The next edition of the State Pharmacopoeia of the Russian Federation, planned for publication in 2015, will include both general pharmacopoeial monographs and pharmacopoeial monographs that were first developed in the practice of domestic and, in some cases, global pharmacopoeial analysis, as well as articles that are updated and revised versions general pharmacopoeial monographs and pharmacopoeial monographs. The introduction of general pharmacopoeial monographs and pharmacopoeial monographs of this edition of the State Pharmacopoeia of the Russian Federation will significantly increase the level of domestic pharmacopoeial analysis and ensure its compliance with the requirements of world standards.
Key words: state pharmacopoeia; general pharmacopoeial monograph; pharmacopoeial monograph; quality of medicines; pharmacopoeial analysis.
Bibliographic description: Merkulov VA, Sakanyan EI, Shemeryankina TB, Mochikina OA, Bunyatyan ND. General pharmacopoeial articles and pharmacopoeial articles of the State Pharmacopoeia of the Russian Federation XIII edition. Gazette of the Scientific Center for Expertise of Medical Products 2015; (2): 54-58.
GENERAL MONOGRAPHS AND PHARMACOPOEIAL MONOGRAPHS OF THE STATE PHARMACOPOEIA OF THE RUSSIAN Federation, xIII Edition v.A. Merkulov, E.I. Sakanyan, T.B. Shemeryankina, O.A. Mochikina, N.D. Bunyatyan
Federal State Budgetary Institution "Scientific Center for Expert Evaluation of Medicinal Products" of the Ministry of Health of the Russian Federation, 127051, Moscow, Russia
Abstract: The State Pharmacopoeia of the Russian Federation is a collection of general monographs and pharmacopoeial monographs. It should be reissued at least once in 5 years. The next scheduled edition of the State Pharmacopoeia of the Russian Federation is planned for publication in 2015. It will include both first developed in national and, in some cases, global pharmacopoeial analysis general and pharmacopoeial monographs, and updated revised general and pharmacopoeial monographs. The implementation of the general and pharmacopoeial monographs of the mentioned edition of the State Pharmacopoeia of the Russian Federation will significantly increase the level of national pharmacopoeial analysis and ensure its compliance with international standards.
Key words: State Pharmacopoeia; general monograph; pharmacopoeial monograph; drug quality; pharmacopoeial analysis.
Bibliographic description: Merkulov VA, Sakanyan EI, Shemeryankina TB, Mochikina OA, Bunyatyan ND. General monographs and pharmacopoeial monographs of the State pharmacopoeia of the Russian Federation, XIII edition. Scientific Center for Expert Evaluation of Medicinal Products Bulletin 2015; (2): 54-58.
The main goal pursued by the State Pharmacopoeia of the Russian Federation (SF RF) is to standardize the quality of medicines in circulation on the domestic pharmaceutical market.
Currently, the State Fund of the X edition (1968), the State Fund of the XI edition (part 1 - 1987, part 2 - 1989), as well as the State Fund of the XII edition (part 1 - 2007) operate on the territory of the Russian Federation. The indicated release dates for these publications of the Global Fund indicate that they do not comply with the requirements of the current Federal Law of April 12, 2010 “On the Circulation of Medicines” No. 61-FZ regarding the timing of the reissue of the State Pharmacopoeia.
Despite this, the general pharmacopoeial monographs (GPM) and pharmacopoeial monographs (PS) included in the State Pharmacopoeia X-X11 editions have not been cancelled. Some of them need revision, some of the articles have already lost their relevance due to lack of demand. These include such GPMs as “Biological method for determining the activity of a 0.1% solution of adrenaline hydrochloride”, “Biological testing
tion of novarsenol and miarsenol”, “Determination of the degree of whiteness of powdered medicines” and others. It is also necessary to abolish the FS for medicines withdrawn from circulation. In addition, the pharmaceutical substances approved during the period between the releases of pharmacopoeias, according to which individual enterprises produce drugs and monitor their quality, need to be revised, since methods of pharmacopoeial analysis are constantly being improved.
Currently, 229 OFS and 179 FS have been prepared for inclusion in the next XIII edition of the State Fund of the Russian Federation. They can be divided into appropriate sections.
The section “General Pharmacopoeial Articles” contains: articles on general methods, General Pharmacopoeial Monographs on methods of analysis, reagents, dosage forms and methods of their analysis; medicinal plant raw materials and methods for assessing their quality; groups of immunobiological drugs and methods of their analysis; medicinal products from human and animal blood and blood plasma and analytical methods used in assessing their quality; radiopharmaceuticals.
Pharmacopoeial monographs are presented in the sections “Pharmaceutical substances” and “Drugs”. The “Pharmaceutical substances” section is presented by pharmacopoeial articles on pharmaceutical substances of synthetic or mineral origin, used as active and/or excipients. In addition, pharmacopoeial monographs for medicinal plant raw materials used in pharmaceutical production, including medicinal herbal preparations, are presented as a separate subsection. The “Medicines” section consists of two subsections: immunobiological drugs and drugs obtained from human blood and plasma.
The appendices to the State Fund of the Russian Federation, XIII edition, are presented with reference tables: a table of atomic masses, alcohol-holometric tables, a table of isotonic equivalents of medicinal substances for sodium chloride, a table of the number of drops in 1 g and 1 ml and the mass of 1 drop of liquid drugs at a temperature of 20 ° C using a standard droplet meter, drawings of IR spectra of standard samples of pharmaceutical substances.
Of this number, for the first time for the State Pharmacopoeia of the Russian Federation, the XIII edition, 102 General Pharmacopoeia Monographs were developed and recommended for approval, including 30 General Pharmacopoeia Monographs for methods of analysis, 5 General Pharmacopoeia Monographs for dosage forms, and 12 General Pharmacopoeia Monographs for methods for determining pharmaceutical and technological parameters of dosage forms, 2 General Pharmacopoeia Monographs for medicinal plant raw materials, and 3 General Pharmacopoeia Monograph for methods of its analysis, 7 General Pharmacopoeia Monograph for groups of immunobiological medicinal products and 31 General Pharmacopoeia Monograph for their testing methods, 3 General Pharmacopoeia Monograph for groups of medicinal products from human and animal blood and plasma, 9 General Pharmacopoeia Monograph for methods of analysis of medicinal products obtained from blood and plasma blood of humans and animals.
In addition, for inclusion in the State Pharmacopoeia of the Russian Federation of the XIII edition, 17 pharmacopoeial articles were prepared for the first time, including 4 FS for pharmaceutical substances, 4 FS for medicinal plant raw materials, 5 FS for immunobiological medicinal products and 4 FS for medicinal products from human blood and blood plasma .
A number of general pharmaceutical substances previously presented in the USSR State Pharmacopoeia in the X and XI editions (USSR State Pharmacopoeia X edition, USSR State Pharmacopeia XI edition) are excluded from the practice of modern pharmacopoeial analysis as unclaimed. Other current OFS and FS State Pharmacopoeia of the USSR X edition, State Pharmacopoeia of the USSR XI edition (issue 1, 2) and the State Pharmacopoeia of the Russian Federation XII edition (SF State Pharmacopoeia of the Russian Federation XII edition) have been revised and supplemented with materials taking into account modern requirements, scientific and practical achievements in the field of pharmacopoeial analysis.
The General Pharmacopoeia Monograph “Rules for the use of pharmacopoeial monographs” has been supplemented with sections “Humidity” and “Storage”. In addition, appropriate clarifications have been made in the sections “Description”, “Mass”, “Volume”, “Temperature”, “Accurate weighing”, “Solvents”, “Indicators”, “Content limits”, “Filtration”.
The OFS “Sampling” includes definitions of terms, general provisions, and the section “Sampling Rules” has been added. New sections have also been introduced: “Sampling from bulk medicines and materials
fishing”, “Sampling of medicinal products in consumer packaging”, “Packaging, labeling, storage of selected samples”, “Requirements for sampling premises, equipment and personnel”.
OFS “Sieve Analysis” was developed to replace the OFS GF XI edition “Determination of grinding of powders and sieves” and indicates the purpose of sieve analysis, conditions and methods for its implementation, classification of typical sieves sizes in accordance with the requirements of world standards.
The new edition of the General Pharmacopoeia Monograph “Sterilization” contains modern current methods and conditions for sterilization of pharmaceutical substances, drugs, excipients, etc., a criterion for the level of sterilization, and a description of biological indicators of sterilization.
In accordance with additional toxicity data, clarifications have been made to the General Pharmacopoeia Monograph “Residual Organic Solvents” and information has been added on solvents with insufficiently substantiated toxicity.
In the General Pharmacopoeia Monograph “Radiopharmaceuticals,” the section “List of quality indicators that radiopharmaceuticals of industrial production and extemporaneous production must meet” has been expanded, and the section “Half-life” has been supplemented with an equation for the half-life curve.
The General Pharmacopoeia Monograph “Pharmaceutical Substances” has made significant additions to the section characterizing the requirements for the quality of pharmaceutical substances (for example, “Residual organic solvents”, “Bacterial endotoxins or pyrogenicity”, etc.). An edited definition of the term “pharmaceutical substance” is provided. The General Pharmacopoeia Monograph has been supplemented with sections on methods of biological analysis: “Abnormal toxicity” and “Histamine and/or Depressor substances”. It includes tables such as “Limits for control, identification and qualification of related impurities for pharmaceutical substances”, “Limits for control, identification and qualification of related impurities in peptides obtained synthetically” and “Criteria for standardizing the permissible content of heavy metals”.
The General Pharmacopoeia Monograph “Shelf life of medicinal products” has been supplemented with the section “Stability tests using the “accelerated aging” method.”
In the General Pharmacopoeia Monograph “General reactions to authenticity” a section “Aluminum” has been additionally introduced, and in the General Pharmacopoeia Monograph “Method of combustion in a flask with oxygen” a section “Selenium” has been added.
The description of tests for purity and permissible limits of impurities in medicines is continued. Thus, for the first time, methods for determining impurities of aluminum, phosphates, mercury and selenium are presented. Methods for determining impurities of ammonium, calcium, arsenic, sulfates, chlorides and zinc and regulatory requirements for their content are harmonized with the requirements of world standards. The General Pharmacopoeia Monograph “Heavy Metals” additionally specifies methods for the quantitative determination of individual ions, and the General Pharmacopoeia Monograph “Iron” contains clarifications regarding the concentration of reagents.
Determination of fluorine in medicines is recommended to be carried out by three methods: titrimetric, spectrophotometric and ionometric.
In addition to the determination of saponification number, acid, ether and iodine numbers, the XIII edition of the State Pharmacopoeia of the Russian Federation includes general pharmacological monographs devoted to the determination of peroxide, hydroxyl and anisidine numbers. Unlike the peroxide value, the anisidine number characterizes the content of secondary oxidation products (aldehydes, ketones) in the tested pharmaceutical substance and/or drug product and thus gives a complete picture of the quality of the drug being analyzed.
The General Pharmacopoeia Monograph “Determination of Protein” has been significantly revised: the structure of the article has been changed, clarification has been made regarding the determination of interfering substances, the description of spectrophotometric and colorimetric methods for determining protein has been expanded, and a fluorimetric method for determining protein using o-phthalaldehyde has been introduced. A method such as the determination of protein with Nessler's reagent is excluded - this method is included in a separate General Pharmacopoeia Monograph "Determination of protein nitrogen with Nessler's reagent with preliminary precipitation of protein material in immunobiological medicinal products."
To characterize the main indicator of the quality of antacid drugs, the 13th edition of the State Pharmacopoeia of the Russian Federation for the first time included the General Pharmacopoeia Monograph “Determination of acid-neutralizing ability.”
Modern spectroscopic methods for studying the structure and quality of drugs are Raman spectrometry, X-ray fluorescence spectrometry, near-infrared spectrometry, infrared spectrometry, ultraviolet and visible spectrophotometry, atomic emission spectrometry, fluorimetry, nuclear magnetic spectroscopy resonance, mass spectrometry, etc. Taking into account the modern capabilities of spectroscopic methods, such OFS as “Raman spectrometry”, “X-ray fluorescence spectrometry”, “Mass spectrometry” and “Near-infrared spectrometry” were first developed.
The need to introduce the OFS “Polymorphism” and “Crystallinity” is due to the relevance of assessing polymorphism and the degree of crystallinity or the content of the amorphous fraction in pharmaceutical substances, which subsequently determines the therapeutic effect of drugs and significantly affects the parameters of their bioavailability.
The General Pharmacopoeia Monograph “Atomic Emission Spectrometry and Atomic Absorption Spectrometry” of the XII edition of the State Pharmacopoeia (Part 1) is divided into two General Pharmacopoeia Monographs: “Atomic Emission Spectrometry” and “Atomic Absorption Spectrometry”. The next edition of the State Fund of the Russian Federation will present a revised and expanded version of the General Pharmacopoeia Monograph “Atomic Emission Spectrometry”.
In the new edition of the General Pharmacopoeia Monograph "Fluorimetry", the wording of the definition of the method has been changed towards greater brevity and versatility, pharmaceutical substances for which this method of determination is available are given, sources of exciting radiation are described, the concept of Stokes shift is given with a brief justification of the cause of this phenomenon, groups of compounds with fluorescent properties-
We have added a list of factors influencing fluorescence intensity.
For the first time, the State Pharmacopoeia includes general pharmacopoeial articles “Optical microscopy” and “Determination of particle size distribution by laser light diffraction.” Unlike the microscopy method, the method for studying dispersed systems based on laser light scattering allows for the assessment of all particles, is non-destructive and allows you to measure particle sizes in the range from 0.1 μm to 3 mm, in contrast to optical microscopy, which is used to characterize particles with sizes from 1 microns or more.
Two separate articles are devoted to weight loss during drying and the determination of water. In addition to the semi-micromethod of K. Fischer, a coulometric method (micromethod) is described, which makes it possible to quantify microquantities of water in medicines.
In the General Pharmacopoeia Monograph “Viscosity” the definition for non-Newtonian liquids has been changed and the conditions for determining viscosity on a rotational viscometer have been characterized. The following subsections have been added as new: “Viscometers with a concentric cylinder (absolute viscometers)”, “Viscometers with a cone-plane system (absolute viscometers)” and “Viscometer with a spindle (relative viscometers)”.
The development of the OFS “Electrical conductivity” was caused by the need to include this quality indicator and the method of its determination in the FS “Purified Water” and “Water for Injections”.
An alternative or additional to chromatographic testing methods is the electrophoresis method. When revising the General Pharmacopoeia Monograph “Electrophoresis”, special attention was paid to the description of the most widely used method in pharmaceutical analysis of protein electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Compared with traditional electrophoresis, the introduction of capillary electrophoresis made possible the automated quantification of not only charged large molecules or microparticles, but cations, anions and neutral compounds.
The polyacrylamide gel electrophoresis method is widely used in separating various proteins and estimating their molecular weight. By varying the concentration of polyacrylamide in the gel, you can control the range of molecular weights of the separated proteins, which is very convenient for obtaining accurate results. Fractionation of protein molecules using this method is widely used to control the quality of protein drugs.
For the first time, automatic elemental analysis is being introduced into the practice of domestic pharmacopoeial analysis, which makes it possible to significantly simplify the analysis of organic compounds containing nitrogen, sulfur, chlorine, bromine, oxygen and other elements. The determination is based on high-temperature oxidative decomposition of the substances under study and subsequent selective determination of the decomposition products corresponding to these elements using gas chromatography. One of the advantages of automatic elemental analysis is the ability to use one standard sample with a known content of the element being determined to assess the quality of various medicines for this element.
Determination of the adsorption activity of enterosorbents is a specific indicator of the quality of this class of drugs. Adsorption activity is used to characterize the absorption capacity of enterosorbents; the methods for its determination are reflected in this General Pharmacopoeia Monograph.
The articles describing biological methods for quality control of medicines included in the XIII edition of the State Fund of the Russian Federation correspond to the modern approach to biological testing. The General Pharmacopoeia Monograph “Bacterial Endotoxins” introduces for the first time a description of photometric methods for determining bacterial endotoxins: turbidimetric and chromogenic.
From the General Pharmacopoeia Monograph “Biological methods for assessing the activity of medicinal plant raw materials and medicinal preparations containing cardiac glycosides,” during its revision, the method of biological assessment of the content of cardiac glycosides carried out on cats was excluded.
The General Pharmacopoeia Monograph “Microbiological purity” has been significantly improved and supplemented with new sections, including those regarding the requirements for immunobiological medicinal products.
The revision of the General Pharmacopoeia Monograph “Determination of the effectiveness of antimicrobial preservatives” made it possible to introduce appropriate additions and clarifications into it regarding the categories of medicines that contain preservatives and the criteria for assessing the effectiveness of antimicrobial preservatives in medicines.
Revision of the General Pharmacopoeia Monograph “Reagents. Indicators" led to a significant increase in the list of reagents and indicators used in pharmacopoeial analysis. Chemical names of reagents and indicators are given in accordance with the requirements of the International Union of Pure and Applied Chemistry (IUPAC). The CAS (Chemical Abstracts Service) registration numbers of chemical substances included in the Chemical Abstracts Service register are indicated. Clarifications and additions have been made to the chemical formulas and physical parameters of reagents and indicators.
When revising the General Pharmacopoeia, “Statistical processing of the results of chemical experiments and biological tests” was divided into two General Pharmacopoeia: “Statistical processing of the results of a chemical experiment” and “Statistical processing of the results of determining the specific pharmacological activity of drugs by biological methods.”
For the first time, the XIII edition of the State Fund of the Russian Federation developed and included such general pharmaceutical substances as “Dosage forms”, “Dosage forms for inhalations”, “Transdermal patches”, “Solutions” and “Cut-pressed granules”.
General Pharmacopoeia Monograph “Dosage Forms” contains basic terms and definitions, classification of dosage forms, general requirements for production/manufacturing, quality assessment, packaging, labeling and storage of medicines in appropriate dosage forms. This General Pharmacopoeia Monograph contains quality indicators that are mandatory for assessing the quality of a medicinal product in any dosage form, as well as quality indicators that characterize the features of the production/manufacturing of the medicinal product and its active and auxiliary substances.
17 General Pharmacopoeia Monograph for dosage forms were introduced to replace the corresponding articles of the State Fund of the USSR, XI edition, with additions and changes made to them.
Most of the General Pharmacopoeia Monographs for methods for assessing pharmaceutical and technological indicators of the quality of dosage forms are included in the 13th edition of the State Pharmacopoeia of the Russian Federation for the first time. Separate OFS were developed for analysis methods previously described in the articles of the State Pharmacopoeia XI for dosage forms (methods for determining the extractable volume of dosage forms for parenteral use, abrasion of tablets, time of complete deformation of lipophilic-based suppositories, disintegration of tablets and capsules).
For the first time developed and included in the State Fund of the Russian Federation in the XIII edition of the General Pharmacopoeia Monograph for such methods for determining pharmaceutical and technological quality indicators of dosage forms as “Visible mechanical inclusions in dosage forms for parenteral use and ophthalmic dosage forms”, “Invisible mechanical inclusions in dosage forms for parenteral use” , “Weight (volume) of the contents of the package”, “Uniformity of dosing”, “Uniformity of the mass of dosage dosage forms”, “Crush strength of tablets”, “Disintegration of suppositories and vaginal tablets”, “Dissolution for lipophilic based suppositories”, “Degree of flowability powders”, “Dissolution for transdermal patches”.
The subsection “Medicinal plant raw materials and methods of its analysis” includes 23 General Pharmacopoeia and 55 FS. Requirements for sampling, storage, packaging, labeling and transportation of medicinal plant raw materials and medicinal herbal preparations are presented in the subsection “General Articles” in the General Pharmacopoeia Monograph “Sampling of medicinal plant raw materials and medicinal herbal preparations”, General Pharmacopoeia Monograph “Storage of medicinal plant raw materials and medicinal herbal preparations” " and General Pharmacopoeia Monograph "Packaging, labeling and transportation of medicinal herbal raw materials and medicinal herbal preparations."
General requirements for medicinal plant raw materials are set out in the General Pharmacopoeia Monograph “Medicinal Plant Raw Materials”. 12 OFS are devoted to methods of analysis of medicinal plant materials and medicinal herbal preparations. 8 General Pharmacopoeia Monographs describe the requirements for methods of analysis of medicinal plant materials depending on morphological groups: flowers, fruits, seeds, buds, herbs, leaves, bark and underground organs. This section also presents 2 General Pharmacopoeia Monographs for medicinal products of herbal origin: General Pharmacopoeia Monograph “Vegetable fatty oils” and “Essential oils”.
The General Pharmacopoeia Monograph “Medicinal Plant Raw Materials” was developed and included for the first time in the State Fund of the Russian Federation. This article provides a classification of medicinal plant raw materials depending on morphological groups, grinding, content of one or another group of biologically active substances, provides the main quality indicators of medicinal plant raw materials and general requirements for storage and packaging.
Of the 12 General Pharmacopoeia Monographs for methods of analysis of medicinal plant raw materials, 3 General Pharmacopoeia Monographs are included in the State Fund of the Russian Federation in the XIII edition for the first time, 9 General Pharmacopoeia Monographs have been revised and introduced to replace the articles of the State Fund of the USSR in the XIth edition. For the first time included in the practice of domestic pharmacopoeial analysis: General Pharmacopoeial Monograph “Determination of the content of heavy metals and arsenic in medicinal plant raw materials and medicinal plants”
medicinal preparations", General Pharmacopoeia Monograph "Determination of the content of residual pesticides in medicinal plant raw materials and medicinal herbal preparations", General Pharmacopoeia Monograph "Determination of the water absorption coefficient and consumption coefficient of medicinal plant raw materials".
The XIII edition of the State Fund of the Russian Federation includes new types of medicinal plant raw materials approved for medical use, such as chokeberry dry fruits, ginkgo biloba leaves, sweet clover grass and poplar buds. The structure of pharmacopoeial monographs for medicinal plant raw materials is harmonized with the requirements of world pharmacopoeial standards for medicinal plant raw materials.
The subsection “Groups of immunobiological medicinal products and methods of their analysis” includes 43 General Pharmacopoeia Monographs and 48 FS for immunobiological drugs.
IMPs include vaccines, toxoids, serums and allergens.
For the first time, the practice of domestic pharmacopoeial analysis introduced OFS for individual groups of ILP, such as “Treatment and prophylactic bacteriophages”, “Pro-biotics”, “Bifid-containing probiotics”, “Colax-containing probiotics”, “Lactose-containing probiotics”, “Spore-containing probiotics” " and "Medicines obtained by recombinant DNA methods."
Of the 48 FS for IMP included in the XIII edition of the State Fund of the Russian Federation, 5 FS were developed for the first time in the practice of domestic pharmacopoeial analysis: “Dysentery vaccine against Shigella Sonne lipopolysaccharide”, “Live cultured rubella vaccine”, “Inactivated smallpox vaccine”, “Human smallpox immunoglobulin” " FS “Pyrogenal, rectal suppositories” was developed for the first time in the practice of domestic and world pharmaceutical analysis.
Medicines from blood and blood plasma of humans and animals are represented by 13 OFS and 8 FS.
Medicinal products from human blood and blood plasma include preparations of human albumin
ka, human immunoglobulin preparations and blood coagulation factor preparations containing one of the blood coagulation factors or a combination thereof.
12 General Pharmacopoeia Monographs for medicinal products from human and animal blood and blood plasma are presented in the XIII edition of the State Pharmacopoeia of the Russian Federation for the first time.
Pharmacopoeial monographs for pharmaceutical substances of synthetic and mineral origin contain the chemical names of medicinal substances in accordance with the requirements of the International Union of Pure and Applied Chemistry (IUP), quality indicators, their standardized values and corresponding methods for analyzing these indicators.
The infrared spectrometry method, which gives the most reliable result, is considered as the main identification method. For a number of substances, the 13th edition of the Appendix to the State Fund of the Russian Federation contains drawings of the IR spectra of standard samples of these pharmaceutical substances.
For quantitative determination, preference is given to classical titrimetric methods of analysis. Along with this, modern methods of physical and chemical analysis are widely used, such as spectroscopy in the ultraviolet region, gas and high-performance liquid chromatography, which involve the use of standard samples. The content of the active substance is given in terms of dry (if weight loss on drying is determined), anhydrous (if water is determined) or an anhydrous substance that does not contain residual organic solvents.
Thus, the introduction into force of the General Pharmacopoeia Monograph and the FS prepared for the next, XIII edition of the State Fund of the Russian Federation will not only cancel or replace obsolete articles of the State Fund of the Russian Federation of previous editions, but will also ensure that the level of domestic pharmacopoeial analysis meets the requirements of world standards.
literature
1. State Pharmacopoeia of the USSR. 10th ed. M.: Medicine; 1968.
2. State Pharmacopoeia of the USSR. 11th ed. Vol. 1. M.: Medicine; 1987.
3. State Pharmacopoeia of the USSR. 11th ed. Vol. 2. M.: Medicine; 1989.
4. State Pharmacopoeia of the Russian Federation. 12th ed. Part 1. M.: Scientific Center for Expertise of Medical Products; 2007.
Federal State Budgetary Institution "Scientific Center for Expertise of Medical Products" of the Ministry of Health of the Russian Federation. Russian Federation, 127051, Moscow, Petrovsky Boulevard, 8.
Merkulov Vadim Anatolievich. First Deputy General Director, Dr. med. sciences, professor.
Sakanyan Elena Ivanovna. Director of the Center for Pharmacopoeia and International Cooperation, Dr. Pharm. sciences, professor.
Shemeryankina Tatyana Borisovna. Head of the Department of State Pharmacopoeia and Pharmacopoeial Analysis, Ph.D. pharm. Sci. Mochikina Olga Alekseevna. Researcher at the Department of State Pharmacopoeia and Pharmacopoeial Analysis.
Bunyatyan Natalya Dmitrievna. Deputy General Director for Research, Dr. Pharm. sciences, professor.
1. The State Pharmacopoeia of USSR. 10th ed. Moscow: Meditsina; 1968 (in Russian).
2. The State Pharmacopoeia of USSR. 11th ed. V. 1. Moscow: Meditsina; 1987 (in Russian).
3. The State Pharmacopoeia of USSR. 11th ed. V. 2. Moscow: Meditsina; 1989 (in Russian).
4. The State Pharmacopoeia of the Russian Federation. 12 th ed. V. 1. Moscow: Federal State Budgetary Institution “Scientific Center for Expert Evaluation of Medicinal Products” of the Ministry of Health of the Russian Federation; 2007 (in Russian).
Federal State Budgetary Institution "Scientific Center for Expert Evaluation of Medicinal Products" of the Ministry of Health of the Russian Federation, 8 Petrovsky Boulevard, Moscow, 127051, Russian Federation.
Merkulov V.A. First Deputy Director General. Doctor of Medical Sciences, professor. Sakanyan EI. Director of Center for pharmacopoeia and international cooperation. Doctor of Pharmaceutical Sciences, professor.
Shemeryankina TB. Head of the Department of State Pharmacopoeia and pharmacopoeia analysis. Candidate of Pharmaceutical Sciences.
Mochikina O.A. Researcher of the Department of State Pharmacopoeia and pharmacopoeia analysis.
Bunyatyan ND. Deputy Director General for Scientific work. Doctor of Pharmaceutical Sciences, professor.