The essence of mass spectrometry. Chromatographic methods and their use in the identification of environmental pollutants
Mass spectrometer
Mass-spectrometer
Mass spectrometer
- a device for determining the masses of atoms (molecules) by the nature of the movement of their ions in electric and magnetic fields.
A neutral atom is not affected by electric and magnetic fields. However, if one or more electrons are taken from it or added to it, then it will turn into an ion, the nature of its motion in these fields will be determined by its mass and charge. Strictly speaking, in mass spectrometers, it is not mass that is determined, but the ratio of mass to charge. If the charge is known, then the mass of the ion is uniquely determined, which means the mass of the neutral atom and its nucleus. Structurally, mass spectrometers can be very different from each other. They can use both static fields and time-varying fields, magnetic and / or electric.
Let's consider one of the simplest options.
A mass spectrometer consists of the following main parts:
a) an ion source, where neutral atoms are converted into ions (for example, under the action of heating or a microwave field) and are accelerated by an electric field, b) areas of constant electric and magnetic fields, and v) of the ion receiver, which determines the coordinates of the points where the ions that crossed these fields fall.
From the ion source 1, the accelerated ions through the slit 2 enter the region 3 of constant and uniform electric E and magnetic B 1 fields. The direction of the electric field is set by the position of the capacitor plates and is shown by arrows. The magnetic field is directed perpendicular to the plane of the drawing. In region 3, the electric E and magnetic B 1 fields deflect ions in opposite directions and the values of the electric field E and the magnetic induction B 1 are selected so that the forces of their action on the ions (respectively qЕ and qvB 1, where q is the charge, and v Is the ion velocity) compensated each other, i.e. was qЕ = qvB 1. At the ion velocity v = E / B 1, it moves without deviating in region 3 and passes through the second slit 4, falling into region 5 of a uniform and constant magnetic field with induction B 2. In this field, the ion moves along a circle 6, the radius R of which is determined from the relation
Мv 2 / R = qvB 2, where М is the mass of the ion. Since v = E / B 1, the mass of the ion is determined from the ratio
M = qB 2 R / v = qB 1 B 2 R / E.
Thus, for a known ion charge q, its mass M is determined by the radius R a circular orbit in region 5. For calculations, it is convenient to use the ratio in the system of units given in square brackets:
M [T] = 10 6 ZB 1 [T] B 2 [T] R [m] / E [V / m].
If a photographic plate is used as an ion detector 7, then this radius will be shown with high accuracy by a black point in the place of the developed photographic plate, where the ion beam fell. In modern mass spectrometers, electron multipliers or microchannel plates are usually used as detectors. The mass spectrometer allows the determination of masses with a very high relative accuracy ΔМ / М = 10 -8 - 10 -7.
Mass spectrometer analysis of a mixture of atoms of different masses also makes it possible to determine their relative content in this mixture. In particular, the content of various isotopes of a chemical element can be determined.
CHELYABINSK STATE UNIVERSITY
Chemical faculty
Course work on the topic
"Mass spectrometric method of analysis"
Completed: student of group X-202
Menshenin A.N.
Checked by: Danilina E.I.
Chelyabinsk
2007
Content
INTRODUCTION
Basics of mass spectrometry
The basic structure of a mass spectrometer
Sample injection methods
Ionization mechanisms
Protonation
Deprotonation
Cationization
Detachment of an electron
Capturing an electron
Ionization methods
Electrospray ionization (ESI)
1. Solvents for electrospray
2. The device of the ionization electrospray
Nanoelectrospray ionization (nanoESI)
Atmospheric Pressure Chemical Ionization (APCI)
Atmospheric pressure photoionization (APPI)
Matrix assisted laser desorption / ionization (MALDI)
3. Advantages and disadvantages of matrix assisted laser desorption / ionization (MALDI).
Desorption / Ionization on silicon (DIOS)
Fast Atom / Ion Bombardment (FAB)
Electronic Ionization (EI)
Chemical ionization (CI)
Comparison of the main characteristics of ionization methods
Mass analyzers
Mass analysis
A brief overview of how analyzers work
Analyzer performance
4. Accuracy
5. Resolution (resolving power)
6. Mass range
7. Tandem mass analysis (MS / MS or MS n)
8. Scan speed
Specific types of analyzers
Quadrupole analyzer
Quadrupole ion trap
Linear ion trap
9. Limitations of the ion trap
Dual focusing magnetic sector
Quadrupole-time-of-flight tandem mass spectrometry
10. MALDI and time-of-flight analysis
Quadrupole time-of-flight mass spectrometry
Fourier transform mass spectrometry (FTMS)
General Comparison of Mass Analyzers Commonly Used with ES
Detectors
Electron multiplier
Faraday Cylinder
Photomultiplier with converting dynode
Matrix detector
Charge (inductive) detector
General comparison of detectors.
Mass spectrometer vacuum
List of used literature
INTRODUCTION
Mass spectrometry has been described as the smallest balance in the world, not because of the size of the mass spectrometer, but because it weighs - molecules. Recently, mass spectrometry has undergone tremendous technological advancement, allowing it to be used for proteins, peptides, carbohydrates, DNA, drugs and many other biologically active molecules. With ionization techniques such as electrospray ionization (ESI) or matrix laser desorption / ionization (MALDI), mass spectrometry has become an indispensable tool for biochemical research.
Basics of mass spectrometry
A mass spectrometer determines the mass of a molecule by measuring the mass-to-charge ratio ( m / z) of its ion. Ions are generated when neutral particles lose or gain charge. After formation, the ions are electrostatically directed to the mass analyzer, where they are separated according to their m / z and finally, they are detected. The result of ionization of molecules, separation of ions and detection of ions is a spectrum, which can be used to determine the molecular weight and even some information about the structure of a substance. An analogy can be drawn between a mass spectrometer and a prism, as shown in rice. 1.1... In a prism, light is divided into components at wavelengths, which are then detected by an optical receptor. Likewise, in a mass spectrometer, the generated ions are separated in a mass analyzer, counted and determined in an ion detector (such as an electron multiplier).
tion, mass analyzer and ion detector. Some instruments combine sample injection and ionization, while others combine a mass analyzer and detector. However, all molecules in the sample are exposed to the same effects regardless of instrument configuration. Sample molecules are introduced through an inlet system. Once inside the device, the molecules are converted to ions in the ionization device and then electrostatically transferred to the mass analyzer. The ions are then separated according to their m / z... The detector converts the energy of the ions into electrical signals, which are then sent to the computer.
Sample injection methods
Sample injection was one of the earliest challenges in mass spectrometry. To analyze the mass of a sample, which is initially at atmospheric pressure (760 Torr), it must be introduced into the device in such a way that the vacuum inside the latter remains practically unchanged (~ 10 -6 Torr). The main methods of sample introduction are direct injection
probe or support, commonly used in MALDI-MS, or direct infusion or injection into an ionization device, as in the ESI-MS method.

Direct introduction: using direct insertion of the probe / support ( rice. 1.3) Is a very simple way of delivering a sample to the instrument. The sample is first placed on the probe and then introduced into the ionization zone of the mass spectrometer, usually through a vacuum valve. The sample is then subjected to the necessary desorption procedures, such as laser desorption or direct heating, to ensure evaporation and ionization.
Direct infusion or injection: A simple capillary or capillary column is used to place the sample in gaseous form or in solution. Direct infusion is also convenient because it allows small amounts of material to be effectively injected into the mass spectrometer without breaking the vacuum. Capillary columns are commonly used to differentiate between separation systems and the ionization device of a mass spectrometer. These systems, including gas chromatography (GC) and liquid chromatography (LC), also serve to separate the various components of a solution that are important for mass analysis. In gas chromatography, the separation of the various components takes place in a glass capillary column. As soon as the sample vapors leave the chromatograph, they are sent straight to the mass spectrometer.

In the 1980s, the impossibility of combining liquid chromatography (LC) with mass spectrometry was due, in large part, to the inability of ionization devices to cope with continuous flow.
LC current. However, electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) now allow LC and mass spectrometry to be combined in routine analysis.
Ionization mechanisms
Protonation – the mechanism of ionization, in which a proton is attached to a molecule, giving it a 1+ charge for each attached proton. Positive charges are usually localized to the main parts of the molecule, such as amines, to form stable cations. Peptides are often ionized by protonation. Protonation is carried out at MALDI, ESI and APCI.
Deprotonation is an ionization mechanism in which a negative charge 1- is obtained when a proton is detached from a molecule. This ionization mechanism is commonly performed with MALDI, ESI and APCI and is very useful for the determination of acidic samples, including phenols, carboxylic acids, and sulfonic acids. The spectrum of negative ions of sialic acid is shown in fig 1.2 .
Cationization- the mechanism of ionization, in which a charged complex is formed during the coordination addition of a positively charged ion to a neutral molecule. In principle, protonation also falls under this definition, therefore, cationization is considered to be the addition of an ion other than a proton, such as an alkali metal or ammonium. In addition, cationization is applicable to molecules that are incapable of protonation. The bond of cations, in contrast to protons, with a molecule is less covalent, so the charge remains localized on the cation. This minimizes charge dilution and molecule fragmentation. Cationization can also be done with MALDI, ESI and APCI. Carbohydrates are the best substances for this ionization mechanism, with Na + as the usual attached cation.
Direct transfer of a charged molecule into the gas phase
The transfer of compounds already charged in solution is easily achieved by using desorption or by ejection of charged particles from the condensed phase into the gas phase. This is usually done using MALDI or ESI.
Detachment of an electron
As the name suggests, electron detachment gives a 1+ molecule a positive charge when an electron is knocked out, so that radical cations are often formed. Observed mainly in electron ionization, electron abstraction is commonly used for relatively non-polar compounds with low molecular weight. It is also known to often lead to the formation of significant amounts of fragment ions.
Capturing an electron
When an electron is captured, a negative charge 1- is imparted to the molecule when an electron is attached. This ionization mechanism is primarily observed for molecules with high electron affinity, such as halogenated compounds.
MASS SPECTROMETRY(, mass spectral analysis), a method for analyzing substances by determining the mass (more often, the ratio of mass to charge m / z) and attributed. quantity obtained during ionization of the investigated substance or already present in the studied mixture. The set of m / z and rel. values of these currents, presented in the form of a graph or table, called. mass spectrum of islands (Fig. 1).The beginning of the development of mass spectrometry was laid by the experiments of J. Thomson (1910), who studied beams of charged particles, the separation of which by masses was carried out using electric. and magn. fields, and the spectrum was recorded at. The first was built by A. Dempster in 1918, and the first mass spectrograph was created by F. Aston in 1919; he also investigated the isotopic. composition of a large number of elements. The first serial was created by A. Nir in 1940; his work laid the foundation for isotope mass spectrometry. Direct connection with gas-liquid (1959) made it possible to analyze complex mixtures of volatile compounds, and the connection with liquid using thermal spray. devices (1983) - mixtures of hardly volatile compounds.
Mac spectral devices.
For dividing the investigated island according to m / z values, measuring these values and currents separated
use mass spectral instruments. Devices in which registration is carried out by electric. methods, called. , and instruments with registration on - mass spectrographs. Mass spectral instruments consist of an input system (inlet system), an ion source, a separating device (mass analyzer), a detector (receiver) that provide a deep enough depth in the entire vacuum system of the instrument, and a control and data processing system (Fig. 2). Sometimes devices are connected to a computer.

Mass spectral devices are characterized by sensitivity, which is defined as the ratio of the number of registered to the number entered. For abs. the sensitivity threshold is taken as min. number of investigated islands (expressed in g,), for relative - min. mass or volume fraction of the islands (expressed in%), to-rye provide registration of the output signal at a signal-to-noise ratio of 1: 1.
Ion source is intended for the formation of gaseous investigated islands and the formation of an ion beam, which is sent further to the mass analyzer. naib. a universal method of ionization of substances - electronic impact. First carried out by P. Lenard (1902). Modern sources of this type are built on the principle of the source of A. Nira (Fig. 3).

Rice. 3. Diagram of an ion source of the A. Nira source type: 1 - permanent magnet; 2 -; 3 - pushing out; 4 - stream; 5 - trap; 6 - ion beam; 7 - input to the islands.
Under the action of a field, the lines of force of which are directed perpendicular to the direction of motion of the ion beam, move along a circular trajectory with a radius of r = (2Vm n / zH 2) 1/2, where V is the accelerating voltage, mn is the mass, z is the charge, H - tension magn. fields. with the same kinetic.
energy, but with different masses or charges pass through the analyzer on decomp. trajectories. Usually, the sweep of the mass spectrum (registration with certain values of m / z) is carried out by changing H at constant V. The spread out of the ion source, in kinetic. energies, as well as imperfect focusing in directions lead to broadening of the ion beam, which affects the resolving power. For static mass analyzer R = r / (S 1 + S 2 + d ), where S 1 and S 2 - resp. the width of the entrance and exit slots, d - beam broadening in the plane of the exit slit. Reducing the size of the slits to increase the resolution of the device is difficult to implement technically and, in addition, leads to very low ion currents; therefore, devices with a large trajectory radius (r = 200 - 300 mm) are usually designed. Resolution m. B. increased also when using mass analyzers with double focusing. In such devices, the ion beam is first passed through a deflecting electric. field special forms, in Krom focusing of the beam on energies is carried out, and then through the magn. the field in which they are focused in directions (Fig. 5).

Rice. 5. Schematic of a mass analyzer with double focusing: S 1 and S 2 - source and detector slits; 1 - capacitor; 2 - magnet.
There are more than 10 types of dynamic. mass analyzers: quadrupole, time-of-flight, cyclotron-resonance, magnetic resonance, radio frequency, farvitron, omegatron, etc. Naib. widely used mass analyzers.
The quadrupole mass analyzer is a quadrupole capacitor (Fig. 6), to the parallel rods of which a constant voltage V and an alternating high-frequency V 0 cos are applied w t (w - frequency, t - time); their sums for each are equal in magnitude and opposite in sign.

Rice. 6. Diagram of a quadrupole mass analyzer: 1 - high-frequency generator; 2 - constant voltage generator; 3 - sweep generator; 4 and 5 - source and detector.
Those emitted from the ion source move in the analyzer chamber along the z axis, parallel to the longitudinal axes of the rods, along complex volumetric spiral trajectories, performing transverse oscillations along the x and y axes. At fixed values of frequency and amplitude, alternating voltages with certain values of m / z pass through
a quadrupole capacitor, y with other values of m / z, the amplitude of the transverse vibrations reaches such a value that they hit the rods and are discharged onto them. The mass spectrum is swept by varying the dc and ac voltage or frequency. For modern. quadrupole R = 8000. The first quadrupole device was built by W. Pauli and H. Steinwedel (Germany, 1953).
The time-of-flight mass analyzer is an equipotential space in which they drift, dividing according to the speed of movement (Fig. 7). generated in the ion source, a very short electric. are "injected" by a pulse in the form of an "ion packet" through the grid into the analyzer. In the process of motion, the initial ion packet is stratified into packets consisting of packets with the same m / z values. The drift velocity of the detached ion packets and, consequently, the time of their flight through the analyzer of length L is calculated by f-le:
![]() (V is voltage). The set of such packets entering the detector forms a mass spectrum. For modern. devices R = 5000 - 10000. The first device was created by A. Cameron and D. Egters (USA, 1948), and in the USSR - by N.I. Ionov (1956).
(V is voltage). The set of such packets entering the detector forms a mass spectrum. For modern. devices R = 5000 - 10000. The first device was created by A. Cameron and D. Egters (USA, 1948), and in the USSR - by N.I. Ionov (1956).

Rice. 7.
Scheme of a time-of-flight mass analyzer: 1 - grid; 2 - detector.
In 1973, BA Mamyrin designed an electrostatic device. reflective mirror, called. mass reflectron.
Cyclotron resonance mass analyzer - a cell in the form of a rectangular parallelepiped or a cube, placed in a homogeneous magnet. field. entering the cell, move in it along a spiral trajectory (cyclotron motion) with a frequency w c = 1/2 p z. H / m, where H is the strength of the magn. fields, i.e., with the same m / z values, have a definite cyclotron frequency. The operation of the device is based on resonant absorption of energy when the field frequency and cyclotron frequency coincide. A method is based on the use of a cyclotron resonance mass analyzer, which is used to determine the mass, in particular, they say. , formed at ion-molecular p-tions in the gas phase; analysis of the structure of high-molecular. ; determination of acid-base sv-in-in. For lungs, R = 10 8. The first ioncyclotron resonance was constructed by G. Sommer, G. Thomas, and J. Heiplom (USA, 1950).
Detectors(receivers) are placed at the outlet of the instrument. Electrometric is used for detection. amplifiers for measuring ion currents up to 10 -
14 A, electron multipliers and scintillation. detectors with a photomultiplier, to-rye ensure the counting of individual (current 10 -
19 A) and have a small time constant, as well as the advantage of which is the possibility of registering all the mass spectrum and signal accumulation.
For the introduction of substances into the ion source, there is a special. system called system of filling. It provides the input of strictly metered quantity in-va, its minim. thermal. decomposition, shortest delivery to the ionization area and automatic. change of samples without violation. The input system and highly volatile materials are cold or heated glass tanks with viscous or mol. leaks, through a cut gaseous in-in
enters the ionization area. When connected with between the ion source and the pier is placed. separator (jet, porous or membrane), in which the carrier gas is removed and enriched with the analyzed substance.
The system for introducing non-volatile substances is most often a vacuum sluice, from which the ampoule with the in-vom is introduced directly into the ionization. camera. The ampoule is fixed on a rod equipped with a heater, with the help of which the necessary t-ra is created for the in-va. In some cases, the ampoule is heated due to the heat of ionization. cameras. To reduce the decomposition of substances, the heating rate is increased, edges must exceed the thermal speed. decomposition. This is how devices that connect a liquid to an ion source operate. Naib. a device based on thermal spraying of a solution of the investigated island is widespread, when it is ionized. Dr. type - a belt conveyor, on a belt to-rogo in-in is delivered to the ion source through a system of locks. When the tape moves, the p-solvent is removed, and in the ion source, with rapid heating of the tape, the medium evaporates and ionizes. In some cases, ionization of the substance is also possible as a result of its bombardment with accelerated particles on the surface of the tape. For hard-volatile nonorg. conn. apply specials. , called. the Knudsen cell. This is a high-temperature crucible with a small diameter hole 0.1-0.3 mm, through which it flows under conditions close to equilibrium.
works in deep (10 -
5 - 10
-
6 Pa and above), which allows you to minimize the loss of resolution due to the collision of the ion beam with neutral ones. The ion source and the mass analyzer have different pumping systems and are connected to each other by a channel of such a size, which is sufficient for the passage of the ion beam. This design prevents a drop in the analyzer when the source rises. The source also requires a high pumping speed to reduce the memory effect (removal of I-in adsorbed on the internal surface of the device). Usually, diffusion devices are created in devices. Turbomolecular ones are also used, providing ultra-high (10 -
7 - 10
-
8 Pa) and pumping out at a speed of several. liters per second; these do not require the use of refrigerated traps.
Data collection and management requires the automation of all processes by means of a computer, which allows to carry out razl. types of studies according to a predetermined program with the conditions of analysis during the operation of the device.
Application of mass spectrometry.
Mass spectrometry is widely used in decomp. fields of science and technology: in and, physics, geology, biology, medicine, in the industry, in paint and varnish and chemical. industry, in the production of ultrapure materials, in nuclear technology, in the village. x-ve and veterinary medicine, for food. industry, in the analysis of pollution products and many others. other Great successes have been achieved in the analysis of biologically important substances; the possibility with the pier is shown. m. up to 15000, with a pier. m up to 45000, etc. Mass spectrometry has found application as an express method in medicine; the principles of mass spectrometry underlie the Naib. feels. leak detectors.
Fatherland. , produced for decomp. purposes, have indices: for the study of the isotopic composition - MI, for the study of chem. composition - MX, for - МС.
Mac-spectrometry allows you to measure the exact mol. mass and calculate the elemental composition of the investigated island, establish the chemical. and spaces. structure, determine the isotopic composition, hold qualities. and
quantities. analysis of complex mixtures org. connections. One of the most important tasks is to find the relationship between the nature of the mass spectrum and the structure of the studied org. ... When ionizing org. a pier is formed. , in which the processes of hetero- and homolytic occur further. rupture of bonds or rupture of bonds with rearrangement and the formation of fragmentation, which, in turn, can undergo further disintegration. Followed. decays established from the mass spectrum are called. directions or ways of decay. Destruction directions are an important characteristic of each class of compounds. The totality of all directions of decay is characteristic for each org. conn. fragmentation scheme. If the mass spectrum is simple, the fragmentation scheme is reduced to a single decay path, for example. at the decay of the pier. CH 3 OH + are successively formed CH 2 = OH + and H — C = O +. In the case of complex mass spectra, the fragmentation scheme corresponds to many, often overlapping, decay directions, for example. fragmentation scheme:

Like. decomposes as a result of breaking the bonds CH-CO, CO-NH, NH-CH and CH-R with the formation of fragmentation acc. And n and X n, B n and Y n, C n and Z n, S n and R n (n is the number of the amino acid residue in the peptide chain), which further disintegrate in the same way. The total number of peaks in such a spectrum can reach several. hundreds. The number of fragments is determined by the structure of the investigated, the supply of internal. energy pier. and fragmentation and the time interval between the formation and its detection. Therefore, when interpreting mass spectra, it is necessary to take into account both the measurement conditions (the energy of the ionizing ones, the accelerating voltage in the ion source, the t-py of the ionization chamber) and the design features of the device. At max. by standardizing the measurement conditions, it is possible to obtain sufficiently reproducible mass spectra. Comparison of the mass spectrum of the system under study with the spectrum available in the catalog - naib. a quick and easy way, in-in in the determination of pollution, control of food products of humans and animals, the study of the processes of drugs. drugs, in forensic science, etc. However, only the mass spectrum cannot be unambiguous, for example. not all isomeric substances form different mass spectra.
Under the conditions of mass spectrometry, some of the excited ones decay after leaving the ion source. Such are called. metastable. In mass spectra, they are characterized by broadened peaks at non-integer values of m / z. One of the methods for studying such - masses and kinetic. energies. The study of the decay of metastable ones is carried out on devices, in which magnets. the analyzer precedes the electrical one. Magn. the analyzer is adjusted so that it misses the metastable
Mass spectrometers devices for separating ionized particles of matter (molecules, atoms) by their masses, based on the effect of magnetic and electric fields on ion beams flying in a vacuum. In M.-S. registration of ions is carried out by electrical methods, in mass spectrographs - by darkening the sensitive layer of a photographic plate placed in the device. M.-s. ( rice. one
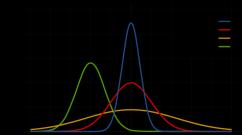
) usually contains a device for the preparation of the test substance 1; ion source 2, where this substance is partially ionized and an ion beam is formed; mass analyzer 3, in which ions are separated by masses, more precisely, usually by the value of the mass ratio m ion to its charge e; ion receiver 4, where the ion current is converted into an electrical signal, which is then amplified and recorded. In addition to information on the number of ions (ion current), the analyzer also receives information on the mass of ions in the recording device 6. M.-s. also contains power supply systems and devices that create and maintain a high Vacuum in the ion source and analyzer. Sometimes M.-s. connected to a computer. For any method of ion registration, the mass spectrum ultimately represents the dependence of the ion current I from m... For example, in the mass spectrum of lead ( rice. 2
) each of the peaks of the ion current corresponds to singly charged ions of lead isotopes. The height of each peak is proportional to the content of a given isotope in lead. The ratio of the ion mass to the width δ m of the peak (in units of mass) R at different levels is also different. So, for example, in the spectrum rice. 2
in the region of the peak of the 208 Pb isotope at a level of 10% relative to the top of the peak R= 250, and at 50% (half-height) R= 380. To fully characterize the resolution of the device, it is necessary to know the shape of the ion peak, which depends on the plural. factors. Sometimes the resolution is called. the value of that maximum mass at which two peaks differing in mass by 1 are resolved to a given level. Since for many types M. - with. R does not depend on the ratio m / e, then both given definitions R match up. It is customary to say that M.-s. With R up to 10 2 has a low resolution, s R Mass spectrometers 10 2 - 10 3 - average, s R Mass spectrometers 10 3 - 10 4 - high, s R> 10 4 - 10 5 - very high. The generally accepted definition of the sensitivity of M. - with. does not exist. If the substance under study is introduced into the ion source in the form of a gas, then the sensitivity of M.-s. often called the ratio of the current generated by the ions of a given mass of a given substance to the partial pressure of this substance in the ion source. This value in devices of different types and with different resolutions lies in the range from 10 -6 to 10 -3 a / mm Hg Art. The relative sensitivity is the minimum content of a substance that can still be detected with the help of M.-s. in a mixture of substances. For different devices, mixtures and substances, it lies in the range from 10 -3 to 10 -7%. For absolute sensitivity, sometimes the minimum amount of a substance in r, which must be entered into M.-s. to detect this substance. Mass analyzers. The classification of M. - S. lies the principle of the device of the mass analyzer. Distinguish between static and dynamic M. - with. Static mass analyzers use electric and magnetic fields to separate ions, which are constant or practically unchanged during the flight of the ion through the device. In this case, the separation of ions is spatial: ions with different values m / e move in the analyzer along different trajectories. In mass spectrographs, ion beams with different values m / e are focused in different places of the photographic plate, forming, after development, traces in the form of stripes (the exit hole of the ion source is usually made in the form of a rectangular slit). In static M.-s. ion beam with a given m / e focuses on the slit of the ion receiver. The mass spectrum is formed (unfolded) when the magnetic or electric field changes, as a result of which ion beams with different magnitudes are sequentially hit into the receiving slit m / e... Continuous recording of the ion current produces a graph with ion peaks ( rice. 2
). To obtain a mass spectrum in this form, recorded by a mass spectrograph on a photographic plate, microphotometers are used.
On the rice. 3
shows a diagram of a common static mass analyzer with a uniform magnetic field. The ions formed in the ion source emerge from the slit of width S 1 in the form of a diverging beam, which in a magnetic field is divided into beams of ions with different moreover, the ion beam with mass m b focuses on the slit S 1 of the ion receiver. The magnitude m b / e defined by the expression: where m b- the mass of the ion (in atomic mass units (see Atomic mass units)) , e- the charge of an ion (in units of an elementary electric charge (See Elementary electric charge)) , r is the radius of the central trajectory of ions (in cm), H- magnetic field strength (in e), V- the applied potential difference (in v), with the help of which the ions in the ion source are accelerated (accelerating potential). The mass spectrum is swept by changing N or V... The first is preferable, since in this case the conditions for "pulling" ions out of the ion source do not change in the course of the sweep. The resolution of such a M.-s .: where σ 1 is the beam width at the point where it enters the receiver slit S 2. If the focusing of ions were ideal, then in the case of a mass analyzer, for which X 1 = X 2 (rice. 3
), σ 1 would be exactly equal to the width of the source slit S 1... In fact, σ 1> S 1, which reduces the resolving power of M. - with. One of the reasons for the beam broadening is the spread in the kinetic energy of ions emitted from the ion source. This is more or less unavoidable for any ion source (see below). Other reasons are: the presence of a given beam of significant divergence, scattering of ions in the analyzer due to collisions with residual gas molecules, "repulsion" of ions in the beam due to the same name of their charges. To weaken the influence of these factors, the "oblique entry" of the beam into the analyzer and the curvilinear boundaries of the magnetic field are used. In some M.-s. use inhomogeneous magnetic fields, as well as the so-called. prism optics (see. Electronic and ion optics). To reduce the scattering of ions, they strive to create a high vacuum in the analyzer (≤10 -8 mmHg cm. in devices with medium and high R-value). To weaken the influence of the spread in energies, M. - with. Is used. dual focusing that focus on the slit S 2 ions with the same m / e flying out not only in different directions, but also with different energies. For this, the ion beam is passed not only through a magnetic field, but also through a deflecting electric field of special shapes ( rice. 4
). Do S 1 and S 2 fewer micron technically difficult. Moreover, this would lead to very low ion currents. Therefore, in devices to obtain high and very high resolution, it is necessary to use large values r and, accordingly, long ion trajectories (up to several m).
In dynamic mass analyzers for the separation of ions with different m / e use, as a rule, different times of flight by ions of a certain distance. There are dynamic analyzers that use a combination of electric and magnetic fields, and purely electrical analyzers. For dynamic mass analyzers, the common thing is the effect on ion beams of pulsed or radio-frequency electric fields with a period less than or equal to the time of flight of ions through the analyzer. More than 10 types of dynamic mass analyzers have been proposed, including time-of-flight (1), radio frequency (2), quadrupole (3), farvitron (4), omegatron (5), magnetic resonance (6), cyclotron resonance ( 7). The first four analyzers are purely electric, while the last three use a combination of permanent magnetic and radio frequency electric fields. During the flight M.-S. ( rice. 5
) ions are formed in the ion source by a very short electrical pulse and are "injected" in the form of an "ion packet" through the grid 1 into the analyzer 2, which is an equipotential space. "Drifting" along the analyzer towards the ion collector 3, the original packet is "stratified" into a number of packets, each of which consists of ions with the same m / e... The stratification is due to the fact that in the initial packet the energy of all ions is the same, and their velocities and, consequently, the time of flight t analyzer are inversely proportional In radio-frequency M. - with. ( rice. 6
) ions acquire the same energy in the ion source eV and pass through a system of successively arranged grid cascades. Each cascade consists of three plane-parallel grids 1, 2, 3, located at an equal distance from each other. A high-frequency electric ω field is applied to the middle grid relative to the two extreme ones U vch. At a fixed frequency of this field and ion energy eV only ions with a certain m / e have such a speed υ that, moving between grids 1 and 2 in a half-period, when the field between them is accelerating for ions, they cross grid 2 at the moment the field changes sign and pass between grids 2 and 3 also in the accelerating field. So they get max. energy gain and fall on the collector. Ions of other masses, passing through these cascades, are either decelerated by the field, that is, they lose energy, or receive an insufficient increase in energy and are discarded at the end of the path from the collector by a high inhibitory potential U 3... As a result, only ions with a certain m / e... The mass of such ions is determined by the ratio: where a- numerical coefficient, S is the distance between the grids. Reconstruction of the analyzer to register ions of other masses is carried out by changing either the initial energy of the ions or the frequency of the high-frequency field. In the quadrupole M. - with. ( rice. 7
) the separation of ions is carried out in a transverse electric field with a hyperbolic potential distribution. The field is created by a quadrupole capacitor (quadrupole), consisting of four rods of circular or square cross-section, located symmetrically about the center, axis and parallel to it. Opposing rods are connected in pairs, and constant and variable high-frequency potential differences are applied between the pairs. The ion beam is introduced into the analyzer along the quadrupole axis through hole 1. At fixed values of the frequency ω and the amplitude of the alternating voltage U 0 only for ions with a certain value m / e the vibration amplitude in the direction transverse to the analyzer axis does not exceed the distance between the rods. Due to the initial velocity, such ions pass through the analyzer and, leaving it through the outlet 2, are recorded, falling on the ion collector. Ions pass through the quadrupole, the mass of which satisfies the condition: where a- constant of the device. The oscillation amplitude of ions of other masses increases as they move in the analyzer so that these ions reach the rods and are neutralized. Reconstruction to the registration of ions of other masses is carried out by changing the amplitude U o or the frequency ω of the variable voltage component. In the farvitron ( rice. eight
) ions are formed directly in the analyzer itself during the ionization of molecules by electrons flying from the cathode, and oscillate along the axis of the device between electrodes 1 and 2. When the frequency of these oscillations ω coincides with the frequency of the alternating voltage U treble, supplied to the grid, the ions acquire additional. energy, overcome the potential barrier and come to the collector. The resonance condition has the form: where a- constant of the device. In dynamic M.-s. with a transverse magnetic field, the separation of ions by masses is based on the coincidence of the cyclotron frequency (see Cyclotron frequency) of ion rotation along circular paths in a transverse magnetic field with the frequency of an alternating voltage applied to the analyzer electrodes. So, in the omegatron ( rice. 9
) under the action of an applied high-frequency electric field E
and constant magnetic field N
ions move along circular arcs. Ions whose cyclotron frequency coincides with the field frequency ω E, move in a spiral and reach the collector. The mass of these ions satisfies the relationship: where a- constant of the device. In magnetic resonance M. - with. ( rice. 10
), the constancy of the time of flight by ions of a given mass of a circular trajectory is used. Ions of close mass from ion source 1 (the region of trajectories of which I
shaded), moving in a uniform magnetic field N
, enter modulator 3, where a thin packet of ions is formed, which, due to the acceleration obtained in the modulator, begin to move in an orbit II
... Further separation in terms of masses is carried out by accelerating "resonant" ions, the cyclotron frequency of which is a multiple of the modulator field frequency. After several revolutions, such ions are again accelerated by the modulator and arrive at the ion collector 2. In cyclotron-resonance M.-s. ( rice. eleven
) resonance absorption of electromagnetic energy by ions occurs when the cyclotron frequency of the ions coincides with the frequency of the alternating electric field in the analyzer; ions move along cycloids in a uniform magnetic field N
with the cyclotron frequency of orbital motion: (With is the speed of light). The resolution for each type of dynamic mass analyzers is determined by a complex set of factors, some of which, for example, the effect of space charge and ion scattering in the analyzer, are common to all types of mass analyzers, both dynamic and static. For devices (1), an important role is played by the ratio of the time during which the ions fly a distance equal to the width of the ion packet to the total time of flight of the ions in the drift space; for devices (3) - the number of ion oscillations in the analyzer and the ratio of the constant and variable components of electric fields; for instruments (5) - the number of revolutions that an ion makes in the analyzer before it hits the ion collector, etc. a high resolution has been achieved: for (1) and (3) R Mass spectrometers 10 3, for (6) R Mass spectrometers 2.5․10 4, for (7) R Mass spectrometers 2․10 3. For M. - with. with a very high resolution, as well as for laboratory instruments of general purpose, from which simultaneously high resolution, high sensitivity, a wide range of measured masses and reproducibility of measurement results are required, the best results are achieved with the help of static M.-s. On the other hand, in some cases, the most convenient dynamic M. - with. For example, time-of-flight M. are convenient for registering processes with a duration from 10 -2 to 10 -5 sec; radio frequency M.-s. due to their low weight, dimensions and power consumption, they are promising in space research; quadrupole M.-s. due to the small size of the analyzer, a large range of measured masses and high sensitivity, they are used when working with molecular beams (see. Molecular and Atomic Beams) .
Magnetic resonance M.-s. due to high R values at low intensity levels, they are used in the geochemistry of helium isotopes to measure very large isotopic ratios. Ionic sources. M.-s. they are also classified according to the methods of ionization, which are used as: 1) ionization by electron impact; 2) photoionization; 3) ionization in a strong electric field (field ion emission) ;
4) ionization by ion impact (ion-ion emission); 5) Surface ionization ;
an electric spark in a vacuum (vacuum spark); 6) ionization under the action of a laser beam (see. Laser radiation).
In analytical mass spectroscopy (see. Mass spectroscopy), due to the relative technical simplicity and rather large generated ion currents, the following methods are most often used: 1 - in the analysis of evaporated substances; 6 - when working with difficult-to-evaporate substances and 5 - during isotopic analysis of substances with low ionization potentials. Method 6, due to the large energy spread of ions, usually requires analyzers with double focusing even to achieve a resolving power of several hundred units. Average ion currents generated by an ion source with electron impact ionization at an ion energy of 40 - 100 ev and the width of the slit of the source Mass spectrometers several dozen micron(typical for laboratory M.-s.), are 10 -10 - 10 -9 a. For other ionization methods, these currents are usually lower. "Soft" ionization, that is, the ionization of molecules, accompanied by a slight dissociation of ions, is carried out with the help of electrons, the energy of which is only 1 - 3 ev exceeds the ionization energy of the molecule, as well as using methods 2, 3, 4. The currents obtained during "soft" ionization are usually Mass spectrometers 10 -12 - 10 -14 a.
Registration of ion currents. The magnitudes of the ion currents generated in the magnetic field determine the requirements for their amplification and registration. The sensitivity of the used in M. - with. amplifiers Mass spectrometers 10 -15 - 10 -16 a at a time constant from 0.1 to 10 sec. Further increase in the sensitivity or speed of the M.-s. is achieved by the use of electron multipliers, which increase the sensitivity of measuring currents in M. - s. up to 10 -18 - 10 -19 amu. Approximately the same sensitivity values are achieved when using photographic registration of ions due to long exposure. However, due to the low accuracy of measuring ion currents and the cumbersomeness of devices for introducing photographic plates into the vacuum chamber of the analyzer, the photo registration of mass spectra retained a certain value only with very accurate mass measurements, as well as in those cases when it is necessary to simultaneously record all lines of the mass spectrum due to instability of the ion source, for example, during elemental analysis in the case of ionization by a vacuum spark. In the USSR, many different mass spectral equipment is being developed and produced. The accepted system of indices for M.-s. classifies devices mainly not by the type of device, but by purpose. The index consists of two letters (MI - M. isotopic, MX - for chemical analysis, MS - for physicochemical, including structural, research, MV - a device with high resolution) and four digits, of which the first indicates the method used to separate ions by masses (1 - in a uniform magnetic field, 2 - in a magnetic inhomogeneous, 4 - magneto-dynamic, 5 - time-of-flight, 6 - radio frequency), the second - on the conditions of use (1 - indicators, 2 - for production, control, 3 - for laboratory research, 4 - for special conditions), and the last two are the model number. On the rice. 12
shows two M.-s. made in the USSR. Abroad M.-s. produced by several dozen companies (USA, Japan, Germany, Great Britain, France and Sweden). Lit .: Aston F., Mass spectra and isotopes, trans. from English, M., 1948; Rafalson A.E., Shereshevsky A. M., mass spectrometric devices, M. - L., 1968; Beynon J., Mass spectrometry and its application in organic chemistry, trans. from English., M., 1964; Materials of the 1st All-Union conference on mass spectrometry, L., 1972; Jeyram R., Mass Spectrometry. Theory and applications, trans. from English, M., 1969; Polyakova A.A., Khmelnitsky R.A., Mass spectrometry in organic chemistry, L., 1972. V. L. Talrose. Rice. 12. On the table of a large mass spectrometer with double focusing for structural and chemical analysis MS-3301 with a resolving power of RMass spectrometers5 · 10 4 there is a miniature MX-6407M mass spectrometer (circled), which was used for studying the ionosphere on artificial earth satellites. Rice. 10. Schematic diagram of a magnetic resonance mass analyzer; a magnetic field N
perpendicular to the plane of the drawing. Rice. 6. Scheme of a radio-frequency mass analyzer: 1, 2, 3 - grids forming a three-grid cascade, a high-frequency voltage U RF is applied to the middle grid 2. Ions with a certain speed and, therefore, a certain mass, being accelerated by a high-frequency field inside the cascade, receive a greater increase in kinetic energy, sufficient to overcome the decelerating field and hit the collector. Rice. 5. Schematic of a time-of-flight mass analyzer. A packet of ions with masses m 1 and m 2 (black and white circles), "thrown" into the analyzer through grid 1, moves in drift space 2 so that heavy ions (m 1) lag behind light ions (m 2); 3 - ion collector. Rice. 4. An example of a mass analyzer with double focusing. The beam of accelerated ions emerging from the slit S 1 of the ion source passes through the electric field of the cylindrical capacitor, which deflects the ions by 90 °, then through the magnetic field deflecting the ions by another 60 °, and is focused into the slit S 2 of the receiver of the ion collector. Rice. 3. Schematic of a static magnetic analyzer with a uniform magnetic field; S 1 and S 2 - slits of the source and receiver of ions; ОАВ - region of uniform magnetic field N
, perpendicular to the plane of the figure, thin solid lines are the boundaries of ion beams with different m / e; r is the radius of the central trajectory of the ions. Rice. 2. Mass spectrum of thorium lead (δm 50% is the peak width at half maximum; δm 10% is the peak width at the level of 1/10 of the maximum intensity). Rice. 1. Skeletal diagram of the mass spectrometer: 1 - system for preparation and introduction of the test substance; 2 - ion source; 3 - mass analyzer; 4 - ion receiver; 5 - amplifier; 6 - recording device; 7 - computer; 8 - power supply system; 9 - pumping devices. The dashed line indicates the evacuated part of the device. Great Soviet Encyclopedia. - M .: Soviet encyclopedia.
1969-1978
.
![]()
![]()
![]()
![]()
![]()











See what "Mass Spectrometers" are in other dictionaries:
mass spectrometers- Devices for the separation of ionizers. particles of a thing (molecules, atoms) by their masses, main. on the influence of magn. and electric. fields on beams of ions flying in vacuum. In m. With. ions register electric methods, in mass spectrographs - by darkening ... ... Technical translator's guide
Mass spectrometers- devices for separating ionized particles of matter (molecules, atoms) by their masses, based on the effect of magnetic and electric fields on ion beams flying in a vacuum. In mass spectrometers, ions are recorded ... ... Encyclopedic Dictionary of Metallurgy
Mass spectrometry capabilities
The mass spectrum can be used to determine the molecular weight of a substance. This is necessary to establish the molecular formula of a substance (gross formula). The mass of an atom, measured with high precision, differs from the mass number. So, for CO 2 and C 3 H 8 the mass number is 44, but their exact relative molecular weights are 43.989828 and 44.062600, respectively, i.e. the difference is 0.072772 amu. The mass spectrometer allows the separation of the beams of CO 2 + and C 3 H 8 + ions when they are obtained simultaneously.
The determination of the atomic composition from the exact value of the mass is carried out using tables of exact masses for various ratios of the number of atoms C, H, O and N as the most common elements. Accurate mass measurement does not replace elemental analysis. Both methods complement each other.
When studying the mass spectrum, in addition to determining the type of molecular ion (M + ) measure peaks for isotopic ions, including lighter or heavier isotopes (with mass numbers M ± 1, M ± 2, M ± 3, etc.). The simultaneous presence of several isotopes in a molecule is unlikely, because the natural abundance of the heavier isotopes C, H, O and N is negligible. For example, 13 C: 12 C = 1 × 10 -2; 2 H: 1 H = 1.6 x 10 -4; 15 N: 14 N = 4 × 10 -3, etc. However, for chlorine 35 Cl: 37 Cl = 3: 1; for bromine 79 Br: 81 Br = 1: 1. Consequently, in the mass spectrum, along with the M + ion will be present (M + 1) + with an intensity proportional to the abundance of isotopes. In widely used look-up tables, the ratios of the intensities of the peaks of molecular ions with mass numbers M + 1 and M + 2 are usually given.
The maximum m / z value in the mass spectrum of a substance can have a molecular ion (M + ), the mass of which is equal to the molecular mass of the test compound. The intensity of the peak of a molecular ion (M +) is the higher, the more stable this ion is.
It is almost rarely possible to establish the complete structure of a compound based on the mass spectrum alone. The most effective is the joint use of several physical and chemical methods. Mass spectrometry, especially in combination with chromatography, is one of the most informative methods for studying the structure of matter (chromatomass spectrometry).
Thus, the possibilities of the method: determination of molecular weight and gross formulas of substances; establishing the structure of a substance by the nature of the resulting fragments; quantitative analysis of mixtures, including the determination of trace impurities; determination of the degree of purity of a substance; determination of the isotopic composition of matter.
Let us consider as an example the mass spectrum of ethanol (Fig. 2). Typically, the spectrum is presented in the form of histograms.
Rice. 2. Mass spectrum of ethanol
In modern devices, the processing of the intensity of electrical pulses corresponding to peaks with different m / z values is performed using a computer.
Mass spectra are recorded as follows: the m / z values are indicated, and the relative intensity (%) in parentheses. For example, for ethanol:
C 2 H 5 OH mass spectrum (m / z): 15 (9), 28 (40), 31 (100), 45 (25), 46 (14).
Interview questions
1. Theoretical foundations of the method.
2. Energy of ionization. Fragmentation types.
3. Schematic diagram of the mass spectrometer.
4. Methods of ionization: electron impact, chemical ionization, etc.
5. Regularities of molecular ion fragmentation.
6. Possibilities of mass spectrometry.
Test tasks
1. Types of molecular ion fragmentation:
a). Dissociation is the disintegration of a molecular ion while maintaining the sequence of bonds. As a result of the process, a cation and a radical are formed, and fragments with even values of the m / z ratio are formed.
Rearrangement - a change in the sequence of bonds, a new radical cation of a smaller mass and a neutral stable molecule are formed, the fragments are characterized by an odd value of the m / z ratio.
b) Rearrangement - the decay of a molecular ion while maintaining the sequence of bonds. As a result of the process, a cation and a radical are formed, and fragments with odd values of the m / z ratio are formed.
Dissociation is a change in the sequence of bonds, a new radical cation of a lower mass and a neutral stable molecule are formed, the fragments are characterized by an even value of the m / z ratio.
c) Dissociation - the disintegration of a molecular ion while maintaining the sequence of bonds. As a result of the process, a cation and a radical are formed, and fragments with odd values of the m / z ratio are formed.
Rearrangement - a change in the sequence of bonds, a new radical cation of a lower mass and a neutral stable molecule are formed, the fragments are characterized by an even value of the m / z ratio.
2. Possibilities of the mass spectrometry method:
a) determination of molecular weight and gross formulas of substances, quantitative analysis of mixtures;
b) establishing the structure of matter by the nature of the resulting fragments, determining the isotopic composition of the matter;
c) determination of molecular weight and gross formulas of substances; establishing the structure of a substance by the nature of the resulting fragments; quantitative analysis of mixtures, including the determination of trace impurities; determination of the degree of purity of a substance; determination of the isotopic composition of matter.
3. Choose the correct answer:
a) The probability of cleavage of the CH bond decreases with an increase in the hydrocarbon chain; the energy of breaking the C-C bond is less; in aromatic derivatives, β-bond cleavage is most likely with the formation of a rearrangement of tropylium ion;
a) The probability of cleavage of the CH bond decreases with an increase in the hydrocarbon chain; the energy of breaking the C-C bond is greater; in aromatic derivatives, β-bond cleavage is most likely with the formation of a rearrangement of tropylium ion;
c) The probability of cleavage of the CH bond decreases with an increase in the hydrocarbon chain; the energy of breaking the C-C bond is less; in aromatic derivatives, the breaking of the a-bond is most probable with the formation of a rearrangement of tropylium ion;
1. Kazin V.N., Urvantseva G.A. Physicochemical research methods in ecology and biology: textbook (stamp UMO) / V.N. Kazin, G.A. Urvantseva; Yarros. state un-t them. P.G. Demidov. - Yaroslavl, 2002 .-- 173 p.
2. Under. ed. A.A. Ishchenko. Analytical chemistry and physicochemical methods of analysis / N.V. Alov et al. - M .: Publishing Center "Academy", 2012. (in 2 volumes, 1 volume-352 p., 2 volume - 416 p.) - (Ser. Bachelor's degree)
3. Vasiliev V.P. Analytical chemistry. - book. 2. Physicochemical methods of analysis. M .: Ministry of Education of the Russian Federation. 2007.383 c.
4. Kharitonov Yu.Ya. Analytical chemistry, vol. 1, book. 2, High School, 2008.
5. Otto M. Modern methods of analytical chemistry (in 2 volumes). Moscow: Technosphere, 2008.
6. Ed. Yu.A. Zolotov. Fundamentals of Analytical Chemistry, Higher School, 2004.
7. Vasiliev V.P. Analytical chemistry. - book. 2. Physicochemical methods of analysis. M .: Bustard, 2009.
8. Kazin V.N. Physicochemical methods of analysis: laboratory practice / V.N. Kazin, T.N. Orlova, I. V. Tikhonov; Yarros. state un-t them. P.G. Demidova. - Yaroslavl: YarSU, 2011. - 72 p.